Busulfan, Melphalan, and Etoposide (BuME) Showed an Equivalent Effect to Busulfan, Cyclophosphamide, and Etoposide (BuCE) as Conditioning Therapy for Autologous Stem Cell Transplantation in Patients with Relapsed or High-Risk Non-Hodgkin’s Lymphoma: A Multicenter Randomized Phase II Study by the Consortium for Improving Survival of Lymphoma (CISL)
Article information
Abstract
Purpose
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard management for relapsed or high-risk non-Hodgkin’s lymphoma (NHL). We reported the busulfan, melphalan, and etoposide (BuME) conditioning regimen was effective in patients with relapsed or high-risk NHL. Moreover, the busulfan, cyclophosphamide, and etoposide (BuCE) conditioning regimen has been used widely in ASCT for NHL. Therefore, based on these encouraging results, this randomized phase II multicenter trial compared the outcomes of BuME and BuCE as conditioning therapies for ASCT in patients with NHL.
Materials and Methods
Patients were randomly assigned to receive either BuME (n=36) or BuCE (n=39). The BuME regimen was comprised of busulfan (3.2 mg/kg/day, intravenously) administered on days −7, −6, and −5, etoposide (400 mg/m2 intravenously) on days −5 and −4, and melphalan (50 mg/m2/day intravenously) on days −3 and −2. The BuCE regimen was comprised of busulfan (3.2 mg/kg/day intravenously) on days −7, −6, and −5, etoposide (400 mg/m2/day intravenously) on days −5 and −4, and cyclophosphamide (50 mg/kg/day intravenously) on days −3 and −2. The primary endpoint was 2-year progression-free survival (PFS).
Results
Seventy-five patients were enrolled. Eleven patients (30.5%) in the BuME group and 13 patients (33.3%) in the BuCE group had disease progression or died. The 2-year PFS rate was 65.4% in the BuME group and 60.6% in the BuCE group (p=0.746). There were no non-relapse mortalities within 100 days after transplantation.
Conclusion
There were no significant differences in PFS between the two groups. Therefore, busulfan-based conditioning regimens, BuME and BuCE, may be important treatment substitutes for the BCNU-containing regimens.
Introduction
High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is the standard treatment for patients with relapsed, refractory, or high-risk non-Hodgkin’s lymphoma (NHL) [1]. Carmustine (bis-chloroethylnitrosourea, BCNU)-containing conditioning regimens such as BCNU/etoposide/cytosine arabinoside/melphalan (BEAM) and BCNU/etoposide/cytosine arabinoside/cyclophosphamide (BEAC) are the most used conditioning regimens for ASCT of lymphoma over the last two decades. With the aim of improving outcomes and reducing toxicities associated with ASCT for lymphoma, several studies on conditioning regimens have been performed [2–4].
Several studies have proposed busulfan, cyclophosphamide, and etoposide (BuCE) as an alternative to BCNU-containing conditioning regimens, which are widely used in current practice [5]. The 3-year progression-free survival (PFS) of patients receiving BuCE was 31%–47%, with an overall survival (OS) rate of approximately 43% at 3 years [5,6]. However, since most of the patients undergoing ASCT have previously received anthracycline-containing chemotherapies, they may be more susceptible to cardiotoxicities from conditioning regimens including high-dose cyclophosphamide. Previous reports have shown that BEAC is a more cardiotoxic regimen than BEAM, due to the differences between cyclophosphamide and melphalan [7,8]. Moreover, the number of elderly patients who undergo ASCT is increasing with improvements in supportive care [9], thereby increasing concerns about cardiotoxicity in ASCT. Thus, we hypothesized that preventing the use of high-dose cyclophosphamide in ASCT may ameliorate the safety levels associated with using conditioning regimens without BCNU.
Recently, the Consortium for Improving Survival of Lymphoma (CISL) reported that administering a busulfan, melphalan, and etoposide (BuME) conditioning regimen prior to ASCT was a well-tolerated and effective treatment for high-risk or relapsed NHL [10]. The 2-year PFS and OS rates for all the patients were 46.7% (95% confidence interval [CI], 31.8% to 60.4%) and 63.7% (95% CI, 47.7% to 76.0%), respectively. There was no development of veno-occlusive disease (VOD) and no treatment-related death within 100 days after ASCT.
Based on these encouraging results, we performed a randomized phase II study to compare the outcomes of patients with relapsed or high-risk NHL receiving BuME and BuCE with ASCT.
Materials and Methods
1. Patients
Patients were enrolled between June 2012 and April 2015. Patients who were 18–65 years old with a biopsy-proven diagnosis of relapsed or primary refractory aggressive NHL, who were sensitive to salvage chemotherapy or had chemosensitive high-risk NHL (two or three risk factors of the age-adjusted international prognostic index) at diagnosis were eligible. Patients were required to have an Eastern Cooperative Group performance status of 0–2 and adequate cardiac, renal, pulmonary, and hepatic functions. Patients with a diagnosis of any other malignancy within the past 5 years except skin basal cell cancer or cervical carcinoma in situ, uncontrolled viral disease (such as hepatitis B), known human immunodeficiency-positive status, serious or uncontrolled systemic disease, or pregnant/breastfeeding were excluded. The study was registered at ClinicalTrial.gov (NCT 03794167).
2. Study design and treatment
This was a prospective, open-labeled, randomized, multicenter phase II study. It was conducted in 17 specialized centers for lymphoma treatment, including ASCT. Based on histologic subtype (B cell vs. T cell), response state at transplantation (complete response [CR] vs. no CR) and response state at transplantation (upfront ASCT vs. refractory/relapsed), the patients were stratified into groups.
Peripheral blood stem cells (PBSCs) were mobilized primarily with granulocyte colony-stimulating factor (G-CSF) alone or with chemotherapy and G-CSF without purge. Hematopoietic stem cells (minimum number, > 2×106 CD34+ cells/kg) were collected from all patients in each participating institution using a large-volume leukapheresis apparatus with a central venous catheter. Cells were cryopreserved with dimethylsulfoxide (DMSO) to achieve a final DMSO concentration of 4.35%–7.5%. The cells were stored at −80°C. Each frozen PBSC product bag was thawed rapidly in a water bath at 40°C and at the patient’s bedside. The frozen PBSCs were infused on day 0.
Eligible patients were randomly assigned to receive either BuME or BuCE as a conditioning regimen of high-dose chemotherapy. The BuME regimen was comprised of busulfan (3.2 mg/kg/day, intravenously), administered on days −7, −6, and −5, etoposide (400 mg/m2 intravenously) on days −5 and −4, and melphalan (50 mg/m2/day intravenously) on days −3 and −2. The BuCE regimen was consisted of busulfan (3.2 mg/kg/day intravenously) on days −7, −6, and −5, etoposide (400 mg/m2/day intravenously) on days −5 and −4, and cyclophosphamide (50 mg/kg/day intravenously) on days −3 and −2. The treatment was administered via a central vein catheter using a controlled-rate infusion pump. Phenytoin (300 mg) was administered orally during intravenous busulfan therapy and 1 day after; it was initiated on the evening before or on the morning of the first dose. ASCT was performed 48 hours after the last dose of melphalan or cyclophosphamide. Patients received care in single rooms, and the protocols for antimicrobial prophylaxis of each participating center were followed. All patients received 5 μg/kg filgrastim or lenograstim daily and subcutaneously after day 3. It was stopped when an absolute neutrophil count (ANC) ≥ 1.5×109/L was achieved for three days.
3. Endpoint
The primary endpoint of the study was 2-year PFS, calculated from the date of transplantation to the date of relapse, progression, or death from any cause. The secondary endpoints were OS and safety. OS was calculated from the day of transplantation to death from any cause. Patients who were alive at the time of the last follow-up were censored. Non-relapse mortality (NRM) was defined as the time from day 0 to death not related to disease recurrence or progression. Engraftment was defined as the first of three consecutive days with an ANC ≥ 0.5×109/L. Platelet engraftment was defined as the first of seven consecutive days with a platelet count of ≥ 20×109/L without transfusion support. Toxicity was classified using the modified National Cancer Institute Common Toxicity Criteria ver. 4.0 (National Cancer Institute, Bethesda, MD). Any non-hematological organ dysfunction from day 0 to day 28 was considered regimen-related toxicity and was graded according to the criteria of Bearman et al. [11].
The patients were followed up by treating physicians every 3 months for the first 2 years after ASCT and every 6 months for 5 years. Tumor responses were assessed using the modified International Working Group criteria [12]. Follow-up examinations after ASCT were conducted every 3 months for 2 years and every 6 months for the next 3 years.
4. Statistical analyses
There was an expected increase in 2-year PFS rates from 40% to 60% with a 5% alpha and 80% power. The calculated minimum sample size of each group was 39; thus, the planned sample size was 78.
For the OS analysis, the Kaplan-Meier method, log rank test, and multivariate Cox proportional hazard model were used. For the multivariate analysis, a stepwise selection procedure was used: all variables that were significant in the univariate analysis (p ≤ 0.1) were included in the multivariate analysis. Hazard ratios and 95% CIs were estimated using a predetermined reference risk of 1.0. Statistical significance was set at p < 0.05. R statistical software ver. 3.5.0 (the R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) were used for all statistical analyses. EZR (ver. 1.41) is a modified version of R commander (http://www.jichi.ac.jp/saitamasct/SaitamaHP.files/statmedEN.html).
Results
1. Patient characteristics
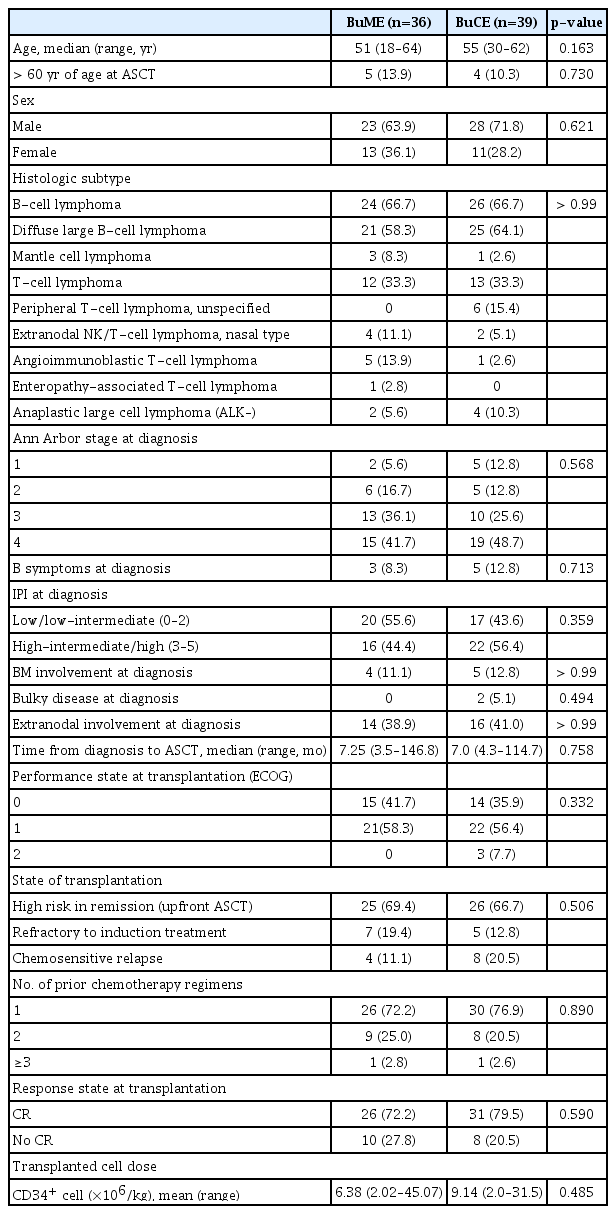
Seventy-eight patients were eligible for this study. However, three patients were excluded: two with disease progression before treatment initiation and one whose hospital become the exclusion center in this study. Thus, 75 patients were enrolled (Fig. 1). Of these, 36 received BuME, and 39 received BuCE. The median age of the patients was 52 years (range, 18 to 64 years). The histologic diagnoses for both groups were: B-cell NHL (n=50, 66.7%) and T-cell NHL (n=25, 33.3%), which included peripheral T-cell lymphoma (n=19, 25.3%), and extranodal natural killer/T-cell lymphoma, nasal type (n=6, 8%). Fifty-one patients (68%) were at high risk for remission and received upfront ASCT. Twelve patients (16%) were refractory to induction therapy but sensitive to salvage chemotherapy, and 12 patients (16%) had chemosensitive relapse. When the demographics, disease, and transplantation characteristics were compared between the conditioning regimen groups (Table 1), there was no significant difference between the two groups.
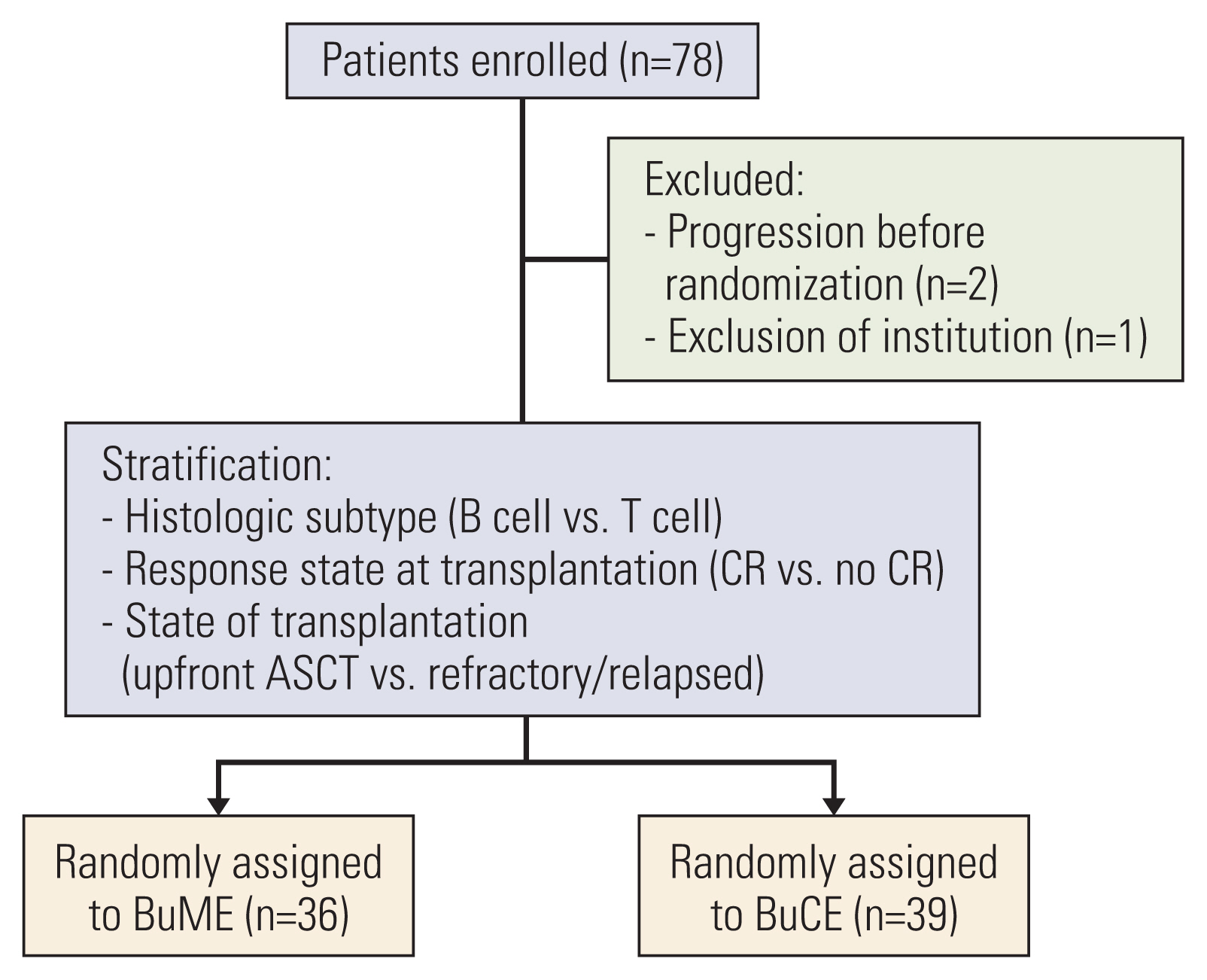
Trial profile. ASCT, autologous stem cell transplantation; BuCE, busulfan, cyclophosphamide, and etoposide; BuME, busulfan, melphalan, and etoposide; CR, complete response.
2. Response and survival
With a median follow-up of 3.75 years, the 2-year PFS and 2-year OS rates in the overall study population were 62.6% (95% CI, 50.3 to 72.6) and 80.2% (95% CI, 68.8 to 87.8) (Fig. 2), respectively.
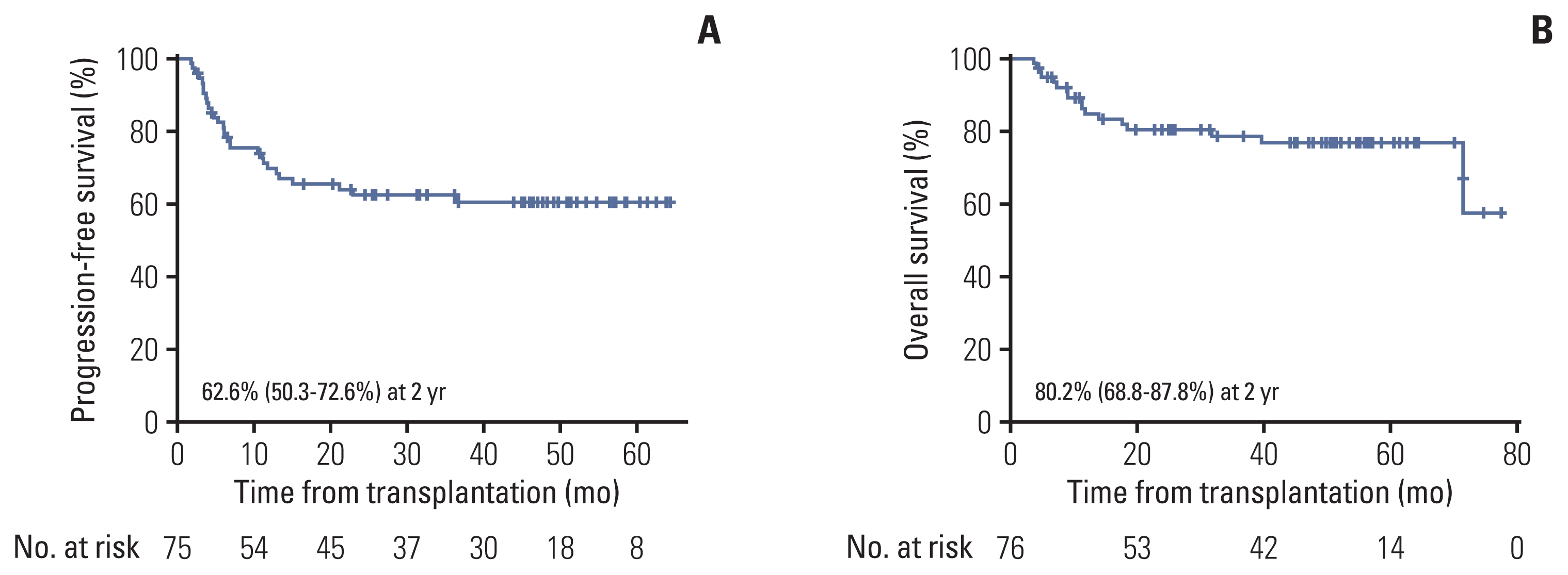
(A) Progression-free survival in overall population (n=75). (B) Overall survival in overall population (n=75).
There was no difference in PFS and OS between the BuME and BuCE groups (Fig. 2). The 2-year PFS rates were 65.4% (95% CI, 47.1 to 78.7) and 60.6% (95% CI, 42.7 to 73.7) for the BuME and BuCE (p=0.746) (Fig. 3A) groups, respectively. The 2-year OS was 85.3% (95% CI, 68.2 to 93.6) and 75.9% (95% CI, 58.8 to 86.7) for the BuME and BuCE groups (p=0.376) (Fig. 3B), respectively.
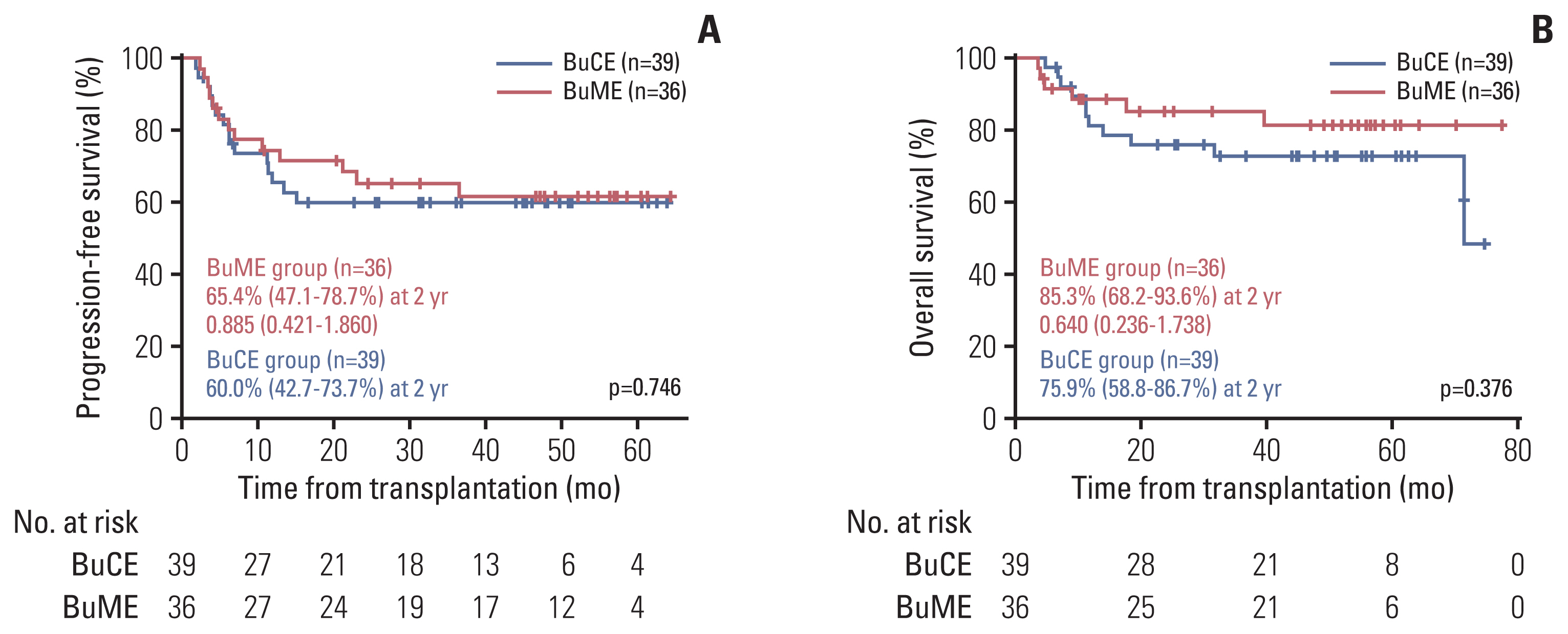
(A) Progression-free survival according to conditioning regimen. (B) Overall survival according to conditioning regimen. BuCE, busulfan, cyclophosphamide, and etoposide; BuME, busulfan, melphalan, and etoposide.
For the patients with disease progression, 22 received salvage treatments (chemotherapy or radiotherapy): 10 in the BuME group and 12 in the BuCE group. Four patients in the BuME group (partial response [PR], 2; CR, 2) and eight patients in the BuCE group achieved a response (4 PR, 4 CR). One patient in the BuME group and four in the BuCE group received allogeneic hematopoietic stem cell transplantation.
3. Engraftment and toxicity
The median dose of transplanted CD34+ cells was 6.38×106/kg (range, 2.02×106/kg to 45.07 ×106/kg) in the BuME group and 9.14×106/kg (range, 2.0×106/kg to 31.5×106/kg) in the BuCE group. The median hospitalization duration was 26.5 (range, 14 to 55 days) and 26 (range, 17 to 68 days) in the BuME and BuCE groups, respectively.
All the 75 patients achieved engraftment of neutrophils (median, day 10; range, 0 to 35) and platelets (median, day 9; range, 0 to 50). The median neutrophil engraftment time was 10 days (range, 0 to 21 days) and 9 days (range, 0 to 35 days) in the BuME and BuCE groups, respectively. The median platelet engraftment time was 11 days (range, 0 to 33 days) and 8 days (range, 0 to 50 days) in the BuME and BuCE groups, respectively.
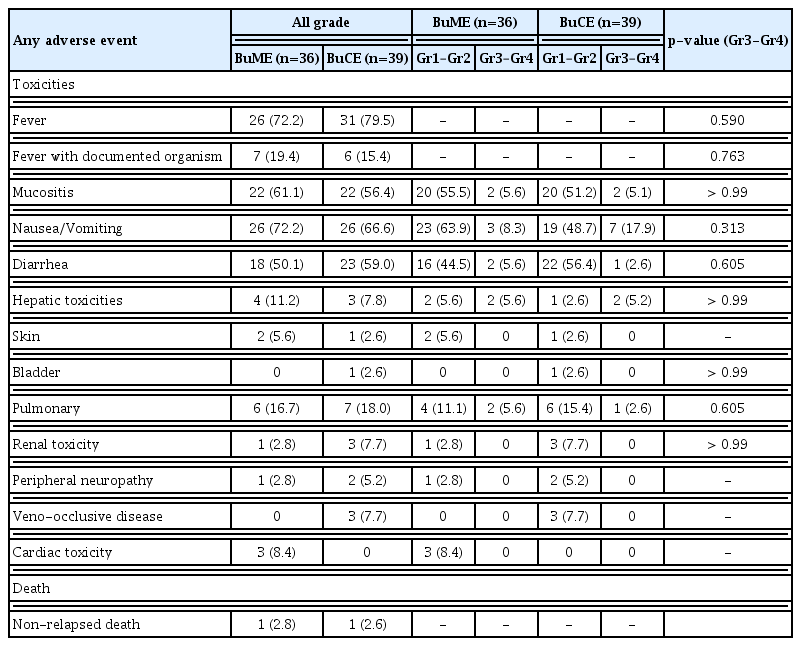
The most frequent nonhematologic toxicities were nausea/vomiting, mucositis, and diarrhea in both groups. Three patients (7.7%) in the BuCE group had mild VOD and recovered completely. There was no VOD in the BuME group. Three patients (8.4%) in BuME group developed grade 1–2 cardiotoxicity, and all of them recovered completely (Table 2).
There was no NRM within 100 days after transplantation in the overall study population. One patient in each group had NRM after 100 days of transplantation. A male patient who received BuME for diffuse large B-cell lymphoma (DLBCL) at the age of 50 years relapsed after 3.8 months of transplantation; he committed suicide 11 months later. He did not receive any treatment after DLBCL relapse. A 52-year-old female patient who received BuCE for DLBCL died from acute hepatitis of unknown origin four months after transplantation.
4. Prognostic factor for survival
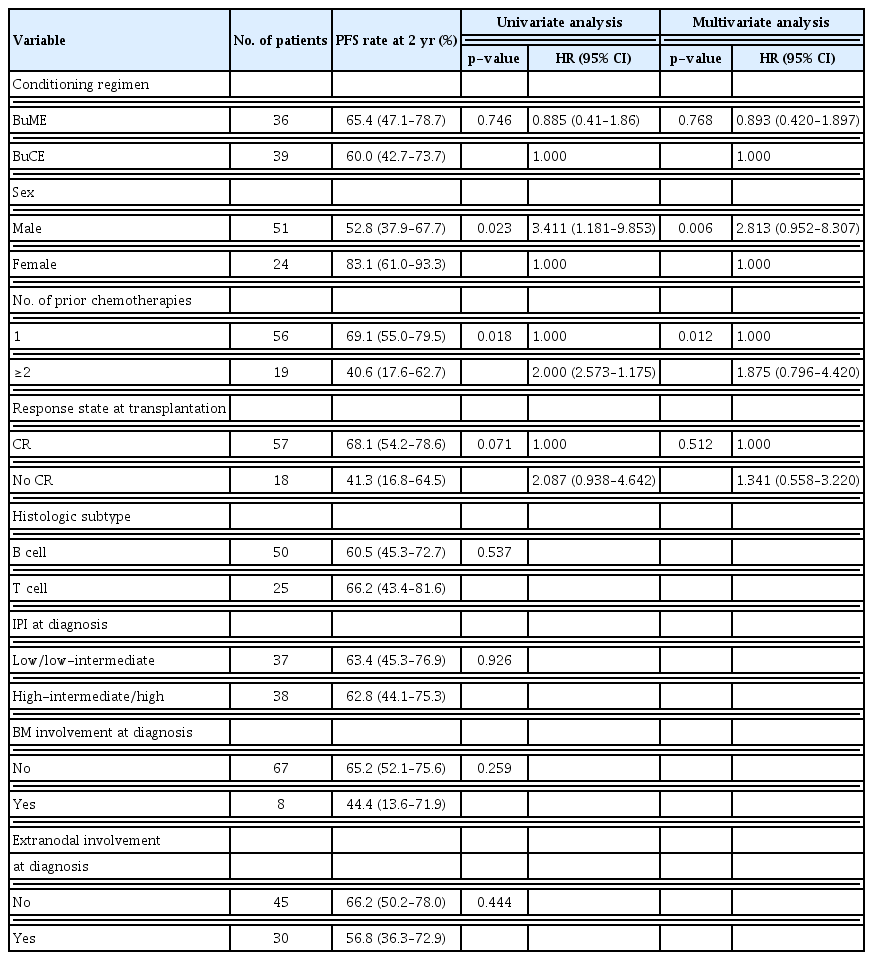
The risk factor analysis for PFS is shown in Table 3. In the univariate analysis, there was no significant difference between the BuME and BuCE groups, but being female, having undergone one prior chemotherapy session were associated with improved PFS.
Being male and having undergone ≥ 2 chemotherapy lines before transplantation were independent risk factors for PFS in the multivariate analyses (Table 3).
Discussion
The 2-year PFS rate was 65.4% in the BuME group and 60.6% in the BuCE group, with no difference between the two groups. The 2-year OS rates were 85.3% and 75.9% in the BuME and BuCE groups, respectively, with no differences between the two groups. BCNU-containing conditioning regimens such as BEAM and BEAC are the most used and accepted standard conditioning regimens in ASCT for lymphoma [2,13]. However, the availability of BCNUs is limited in some countries. Therefore, our working group (CISL) performed several studies to develop a conditioning regimen to replace BCNU-containing regimens. In the previous phase II study of the BuME conditioning regimen that we performed, the BuME conditioning regimen when administered prior to ASCT was well-tolerated and effective for patients with high-risk or relapsed NHL [10]. Thus, in this study, we compared BuME and BuCE. We found that BuME and BuCE were effective conditioning regimens. No significant differences were observed in PFS between the BuCE and BuME groups. This is the first study to compare two busulfan-containing conditioning regimens.
The outcomes of both busulfan-containing conditioning regimens in this study and BCNU-containing conditioning regimens were similar; however, given that this was a direct comparative study, it cannot be regarded as a conclusion [2,9]. In the European Group for Blood and Marrow Transplantation (EBMT) registry report, the 2-year PFS rate was 63% (95% CI, 58 to 69) for patients who received BEAC and 64% (95% CI, 60 to 68) for those who received BEAM (p=0.91) [13]. These results were similar to those of studies on other non-carmustine alternative conditioning regimens. In a study wherein carmustine was replaced with bendamustine in a Benda-EAM (or BeEAM) regimen, Redondo et al. reported a 3-year PFS of 58% and OS of 75% [14]. Moreover, although we included a larger number of patients with T-cell lymphoma and an unfavorable prognosis in this study, the survival outcomes were similar to those in the study by Redondo et al. [14].
HDT followed by ASCT is the standard treatment for patients with relapsed, refractory, or high-risk NHL [1]. However, the major shortcoming of transplantation is the associated severe nonhematologic toxicities, with transplant-related mortality rates of 1%–5%. In an EBMT registry study of BEAM and BEAC, at 100 days, at 1-year and 2-year cumulative incidence of NRM was 2% (95% CI, 1 to 4), 4% (95% CI, 2 to 6), and 5% (95% CI, 3 to 7), respectively, for patients who received BEAC; and 3% (95% CI, 2 to 4), 3% (95% CI, 2 to 4), and 3% (95% CI, 2 to 5) for patients who received BEAM (p=0.27) [13]. There was no NRM within 100 days after transplantation in the overall population of this study. All the patients engrafted to achieve neutrophil and platelet counts of > 0.5×109/L and > 20×109/L, respectively. These results suggest that both BuME and BuCE are well-tolerated regimens.
In our previous study, we reported that a conditioning regimen with busulfan, melphalan, and thiotepa seemed to be too toxic and associated with grades 3–4 mucositis (65%) and VOD (46%). The main cause of the toxicity was presumed to be triple-alkylating drugs [15]. Therefore, we planned subsequent trials with more caution regarding toxicities. After modification of the included drugs, we conducted a phase II multicenter study of BuME [10]. Similar to this study, no case of VOD was registered in the BuME phase II study. In previous studies on busulfan, cyclophosphamide (BuCy), there have been concerns about VOD, which is the commonest registered life-threatening toxicity [16]. In vitro, busulfan and cyclophosphamide metabolites deplete hepatic glutathione levels, and thereby inducing oxidative stress and hepatotoxicity [17]. High-dose cyclophosphamide may significantly increase the risk of developing VODs [16]. Thus, newer conditioning regimens were evaluated, including those with lower busulfan doses and/or an addition of other medications, such as etoposide [18]. The incidence of severe VOD was reduced by 3%–10%. Kim et al. [5] reported mild VOD (4.7%) and moderate VOD (1.6%) in a retrospective study on BuCE conditioning. In our study, three patients in the BuCE group had VOD; one of them had VOD with a concurrent infection. However, all patients had mild to moderate VOD, and they recovered fully. Another major non-hematologic toxicity, cardiotoxicity, is a common complication associated with high-dose cyclophosphamide. BEAC is generally more cardiotoxic than BEAM because of the differences between cyclophosphamide and melphalan [8]. Based on this, we hypothesized that BuCE would be more cardiotoxic than BuME. However, no cardiotoxicity was observed in the BuCE group. Three patients in the BuME group had grades 1–2, and 2/3 patients had a concurrent infection. All the patients recovered fully. Melphalan as well as cyclophosphamide is arrhythmogenic chemotherapeutic agent [19]. Our results confirmed that BuME and BuCE were relatively safe conditioning regimens; however, they had slightly different toxicity profiles. Therefore, a more careful baseline comorbidity assessment of patients is required with a reliable predictive marker of serious toxicity.
Recently, several studies have assessed the effects of adding novel agents to conditioning regimens on outcomes. In a Center for International Blood and Marrow Transplant Research (CIBMTR) registry analysis of 862 patients with DLBCL who had received first-line therapy (BEAM [n=667] or R-BEAM [rituximab plus BEAM; n=195] conditioning) between 2003 and 2017, the addition of rituximab to the conditioning regimen was not beneficial [20]. Maintenance rituximab after transplantation did not improve the event-free survival in CORAL, and neither did substitution of radioimmunotherapy for rituximab as part of the BEAM conditioning regimen in the randomized phase III BMT CTN 0401 study [21]. Several studies on ASCT for T-cell lymphoma have used a modified combination of cytotoxic drugs and have shown similar results [22–24]. Thus, in high-dose chemotherapy and ASCT for NHL treatment, the efficacy of conditioning chemotherapy may be constant. To maximize efficacy, we should determine the ASCT and specific conditioning regimen by considering regimen-related toxicities in accordance with comorbidities, histologic subtypes, biological subtypes, chemosensitivities, and residual tumor burden [25]. The results of this study are supportive and provide evidence for the choice of two feasible busulfan-containing regimens.
However, this study has several limitations. First, this was a phase II trial with a small sample size, despite its randomized design. Second, we could not compare busulfan-containing conditioning regimens with the most used BEAM regimen. A phase III comparative study is underway. Third, the study population was heterogeneous e.g., different clinical settings and tumor histology; the treatment outcome of NHL depends on these factors. Many studies are reporting the effectiveness of new treatment modalities such as chimeric antigen receptor T cell [26] and novel targeting agents [27]. Furthermore, clinical trials of ASCT with a homogenous population are needed.
In conclusion, BuME had a similar efficacy and toxicity with BuCE. Thus, both BuME and BuCE may good treatment substitutes for BCNU-containing conditioning regimens.
Notes
Ethical Statement
The study complied with the principles of the Declaration of Helsinki and guidelines of good clinical practice. The institutional review board or ethics committee at each participating center approved the study protocol and its amendments. Written informed consent was obtained from all the patients.
Author Contributions
Conceived and designed the analysis: Kim KH, Won JH.
Collected the data: Kim KH, Lee JH, Lee M, Kim HG, Do YR, Park Y, Shin HJ, Kim WS, Park SK, Kong JH, Park MR, Yang DH, Kwak JY, Kang HJ, Mun YC, Won JH.
Contributed data or analysis tools: Kim KH, Lee JH, Lee M, Kim HG, Do YR, Park Y, Oh SY, Shin HJ, Kim WS, Park SK, Kong JH, Park MR, Yang DH, Kwak JY, Kang HJ, Mun YC, Won JH.
Performed the analysis: Kim KH, Won JH.
Wrote the paper: Kim KH, Won JH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported, in part, by the Soonchunhyang University Research Fund.