Real World Evidence of Neoadjuvant Docetaxel/Carboplatin/Trastuzumab/Pertuzumab (TCHP) in Patients with HER2-Positive Early or Locally Advanced Breast Cancer: A Single-Institutional Clinical Experience
Article information
Abstract
Purpose
Docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP) regimen is frequently used to treat early and locally advanced human epidermal growth factor receptor 2 (HER2)–positive breast cancer (BC) in neoadjuvant setting. However, large-scaled real-world evidence did not exist.
Materials and Methods
We retrospectively reviewed medical records of patients with early or locally advanced HER2-positive BC who underwent neoadjuvant TCHP followed by curative surgery at Samsung Medical Center between January 2016 and August 2020.
Results
Of 447 patients, 316 (70.7%) received breast-conserving surgery and 131 (29.3%) received total mastectomy. In terms of neoadjuvant chemotherapy response, pathologic complete response (pCR) and residual cancer burden (RCB) score were analyzed. The rate of pCR was 64% a class of RCB 0 was observed in 65% of cases, RCB class I in 12%, RCB class II in 14%, and RCB class III in 2%. The 3-year event-free survival rate was 90.6%, BC with pCR occurred in 92.8%, and BC with non-pCR in 86.3% (p=0.016). In terms of distant metastasis, the 3-year distant recurrence-free survival rate was 93.5%; BC with pCR occurred in 95.9% and BC with non-pCR in 89.2% (p=0.013). Mucositis (85.2%), pain (83.2%), and diarrhea (70.5%) were the most common non-hematologic adverse events. In terms of hematologic adverse events, anemia (89.9%) was the most commonly observed adverse events followed by thrombocytopenia (29.8%).
Conclusion
Neoadjuvant TCHP therapy had a pCR rate of 64% and a 3-year event-free survival of 90% in real world experience. In terms of toxicity profile, anemia was frequently observed and adequate management including occasional transfusion was required.
Introduction
Anti–human epidermal growth factor receptor-2 (HER2) monoclonal antibody, trastuzumab improved survival of patients with early and advanced HER2-positive breast cancer (BC) [1,2]. In terms of neoadjuvant setting, adding trastuzumab in cytotoxic chemotherapy improved pathologic complete response (pCR) and event-free survival (EFS) [3].
The addition of pertuzumab to trastuzumab and cytotoxic chemotherapy significantly improved overall survival in HER2-positive metastatic BC [4,5]. Subsequently, many clinical trials have demonstrated successful outcomes with pertuzumab and trastuzumab combination in HER2-positive BCs regardless of treatment setting [4,6–8].
As neoadjuvant therapy, pertuzumab added to trastuzumab and cytotoxic chemotherapy improved pCR and patient survival [7,9]. Accordingly, treatment guidelines for HER2-positive early or locally advanced BC have been established with trastuzumab±pertuzumab and chemotherapy as the standard treatment strategy [10,11].
Among clinical trials with pertuzumab for early or locally advanced HER2-positive BC, the TRYPHAENA clinical trial was designed to evaluate the safety and efficacy of pertuzumab and trastuzumab in combination with anthracycline- or carboplatin-based neoadjuvant chemotherapy (NAC) in HER2-positive BC [12]. The reported safety profile in this study indicated that six cycles of docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP) had severe adverse events (SAEs) in approximately 30% of cases. This represents the highest rate of SAEs compared with other treatment arms despite lowest cardiac toxicity [12]. Approximately 70% of patients experienced diarrhea, and grade 3/4 neutropenia was observed in about 50% of patients who received the neoadjuvant TCHP regimen [12]. In terms of efficacy, TCHP had a pCR of 64% and 90% 3-year disease-free survival (DFS) [12,13].
Recent advance of adjuvant treatment strategy suggested that trastuzumab emtansine (T-DM1) significantly improved 3-year DFS in HER2-positive BC which did not achieved pCR after NAC compared with trastuzumab treatment despite several toxicities [14]. Therefore, pCR achievement is the important factor to decide adjuvant treatment strategy as well as surrogate marker of survival. Now, TCHP regimen is frequently used for HER2-positive BC in neoadjuvant settings because of the best in terms of pCR.
Here, we report our clinical experience with BC patients treated with neoadjuvant TCHP followed by curative surgery. Comprehensive analysis of the efficacy and safety of the neoadjuvant TCHP regimen were performed in real world experience (RWE).
Materials and Methods
1. Patients
We retrospectively reviewed electronic medical records of patients with early or locally advanced HER2-positive BC who underwent neoadjuvant TCHP chemotherapy followed by curative surgery at Samsung Medical Center between January 2016 and August 2020. We included patients diagnosed with clinical stage II to IIIC BC by diagnostic examinations (breast ultrasonography and/or magnetic resonance imaging, chest and abdomino-pelvic computed tomography (CT) scan, bone scan, and/or positron emission tomography–CT scans, if indicated). Stage was based on American Joint Committee on Cancer (AJCC) 7th edition. Patients who received previous BC surgery due to local recurrence after curative surgery or who underwent palliative surgery were excluded. In the event of bilateral BC, one BC that required NAC was selected.
2. Neoadjuvant chemotherapy
Patients received six cycles of TCHP neoadjuvant therapy. The study drugs were administered intravenously once every 3 weeks. Details of administration method were described in previous article [12]. Prophylactic pegfilgrastim was administered at every treatment cycle.
3. BC pathology
Pathologists determined BC histology and receptor status (estrogen receptor [ER], progesterone receptor [PR], and HER2) according to hematoxylin and eosin and immunohistochemical (IHC) staining. ER and PR positivity were defined as Allred score in the range of 3–8 according to IHC staining with antibodies to ER (Immunotech, Marseille, France) and PR (Novocastra, Newcastle upon Tyne, UK), respectively. HER2 status was evaluated using the appropriate antibody (DAKO, Carpinteria, CA) and/or silver in situ hybridization (SISH). HER2 grades of 0 and 1 were defined as negative results, while grade 3 was identified as a positive result. HER2 amplification was confirmed by SISH results of 2+. All HER2-positive BC were included regardless of ER and PR state.
After surgery, pathologic response to NAC was determined as pCR or residual cancer burden (RCB) [15]. We defined pCR as no residual invasive tumor in the primary tumor bed and ipsilateral axillary lymph nodes regardless of the presence of ductal carcinoma in situ (DCIS) (ypT0/is ypN0) [16].
4. Statistical analysis
EFS was defined as the elapsed time from date of curative surgery to detection of local or distant tumor recurrence. We also included contralateral or ipsilateral DCIS as an observed event. Distant recurrence-free survival (DRFS) was defined as the elapsed time from date of curative surgery to detection of distant metastasis. Overall survival (OS) was defined as the duration between curative surgery and death. DRFS and OS were analyzed using the Kaplan-Meier method. Cox proportional-hazard regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Two-tailed p-values < 0.05 were considered statistically significant, and IBM SPSS Statistics ver. 21 (IBM Corp., Armonk, NY) was used for analysis of all data.
Results
1. Patient cohort
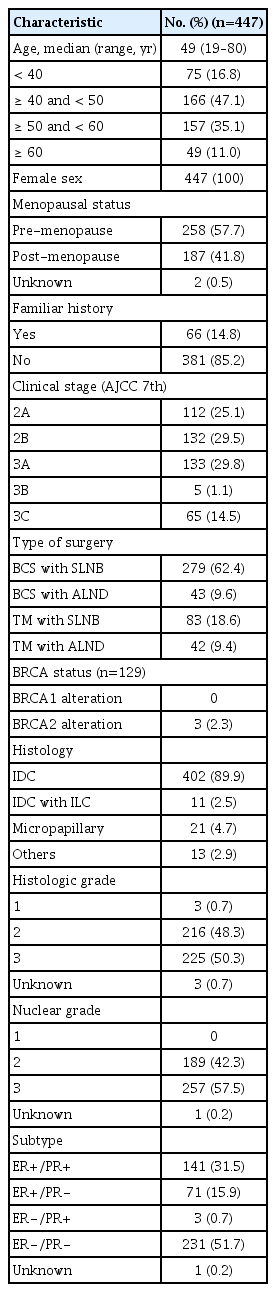
Between February 2016 and August 2019, 1,840 BC patients received NAC followed by curative surgery. Among these patients, those with HER2-positive BC numbered 539 (38.0%), and the TCHP regimen was used in 447 (24.3%) (S1 Fig.). Baseline characteristics of these 447 patients are described in Table 1. Hormone receptor positivity was observed in 48.3% of these patients, and 45.4% were stage III. Median age of patients was 49.
2. Response to neoadjuvant TCHP regimen
Of 447 patients, 279 (62.4%) received breast-conserving surgery (BCS) with sentinel lymph node biopsy (SLNB), 43 (9.6%) received BCS with axillary lymph node dissection (ALND), 83 (18.6%) opted for total mastectomy (TM) with SLNB, and 42 (9.4%) underwent TM with ALND. When axillary lymph node metastasis was suspected, fine needle aspiration was performed at the time of BC diagnosis. We confirmed axillary lymph node metastases pathologically in 172 patients. Of these 172 patients, ALND was performed in 47 (27.3%). Twenty-eight patients (16.3%) had axillary lymph node metastasis at the time of curative surgery.
In terms of NAC response, we evaluated pathologic response at the time of curative surgery. This evaluation included pCR and RCB score. The rate of pCR was 64% and differed according to hormone receptor status (p < 0.001), clinical stage (p=0.028), and histologic grade (p=0.010) (Fig. 1). Other factors that affected NAC response are described in S2 Table. In this analysis, relative dose intensities (RDIs) of docetaxel and carboplatin did not affect NAC response (p=0.187 and 0.917, respectively). In terms of RCB score, a class of RCB 0 was observed in 65% of cases, RCB class I in 12%, RCB class II in 14%, and RCB class III in 2% (S3 Table). Four cases did not achieve pCR but in RCB class 0 because lymphovascular invasions remained in two cases, lymphatic emboli in one case and isolated tumor cell in lymph node in one case after NAC.
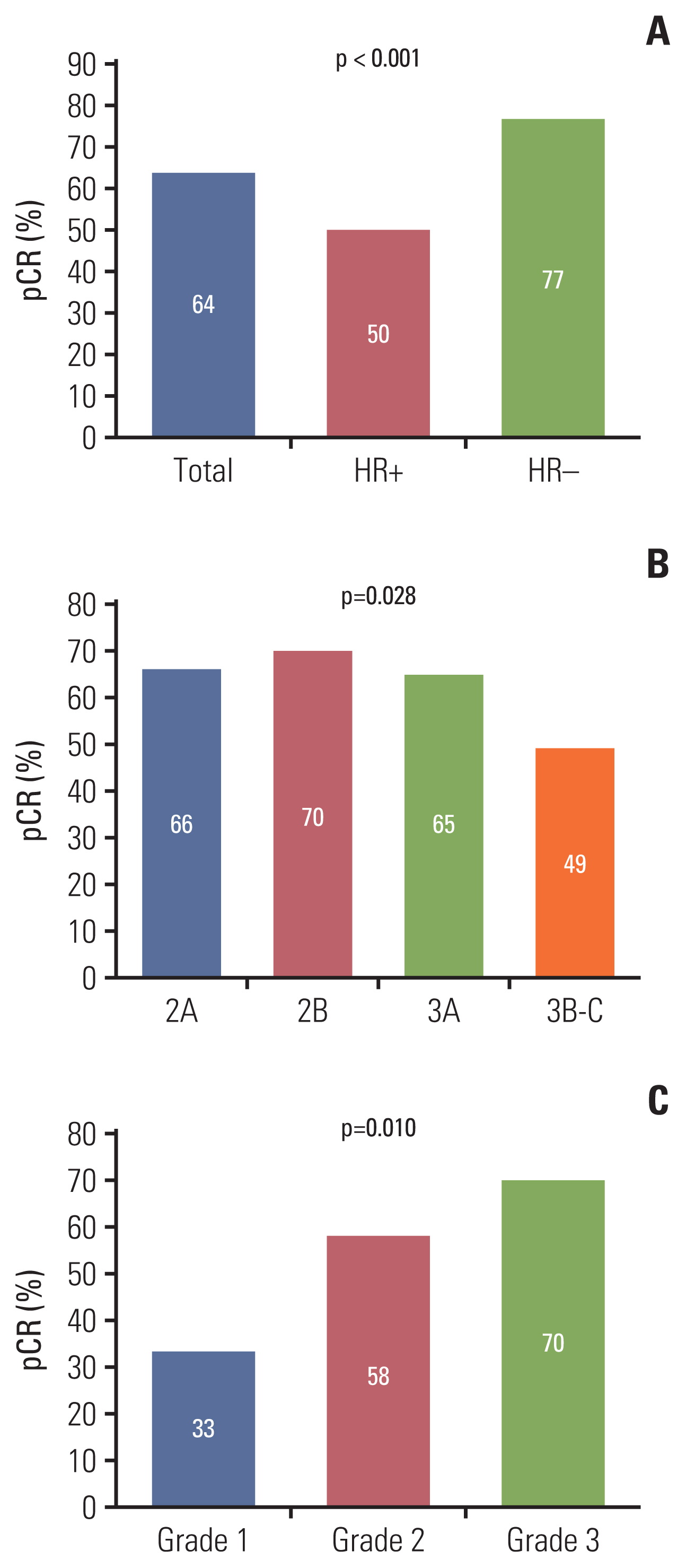
Pathologic complete response (pCR) according to hormone receptor (HR) status (A), clinical stage (B), and histologic grade (C).
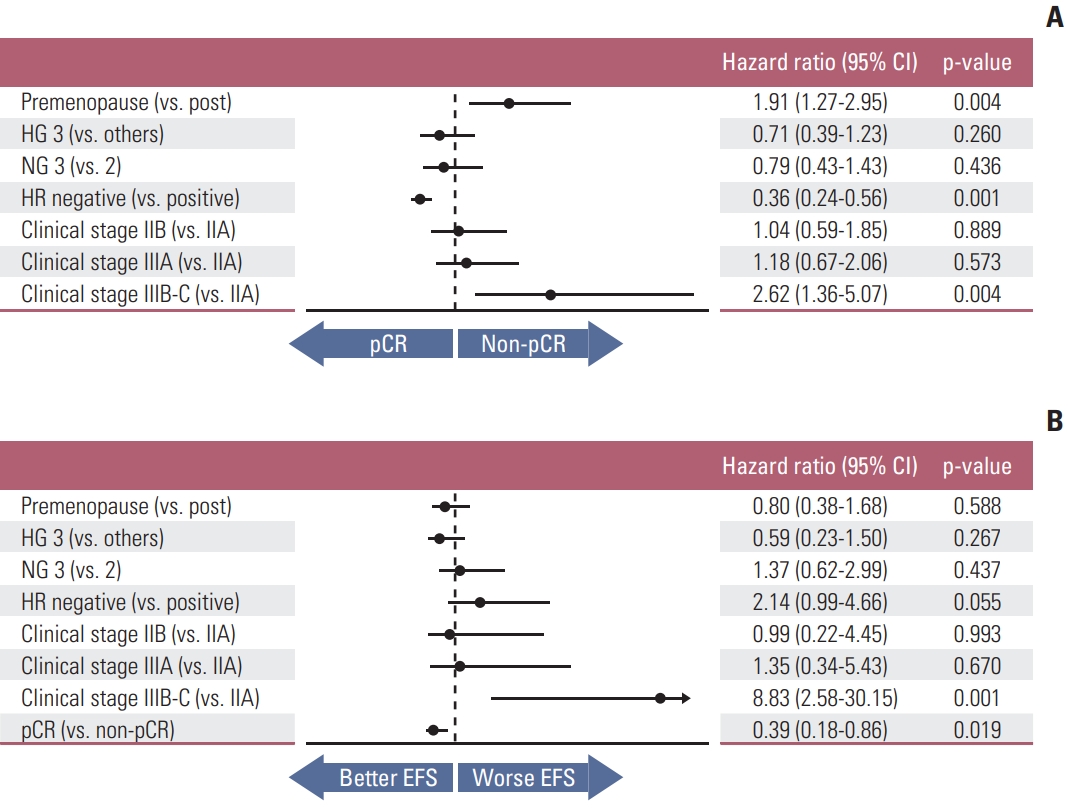
In multivariate analysis of the associations between baseline characteristics and pCR status, hormone receptor negativity was positively related to pCR (HR, 0.36; 95% CI, 0.24 to 0.56; p=0.001) whereas pre-menopausal status and advanced clinical stage were oppositely (HR, 1.91; 95% CI, 1.27 to 2.95; p=0.004 and HR, 2.62; 95% CI, 1.36 to 5.07; p=0.004, respectively) (Fig. 2A).
3. Survival analysis
We ceased data acquisition in December 2020, and the median follow-up duration was 36 months. During follow-up, 33 events have occurred, 24 cases of distant metastasis and nine of local recurrence. The 3-year EFS rate was 90.6%, BC with pCR occurred in 92.8%, and BC with non-pCR in 86.3% (p=0.016) (Fig. 3). In terms of distant metastasis, the 3-year DRFS rate was 93.5% in the total patients, 95.9% in pCR patients, and 89.2% in non-pCR patients (p=0.013). There were two deaths, and the 3-year OS rate was 99.6%. Both deaths were in the non-pCR group. OS was also grouped according to pCR status, but significant difference was not observed (p=0.059).
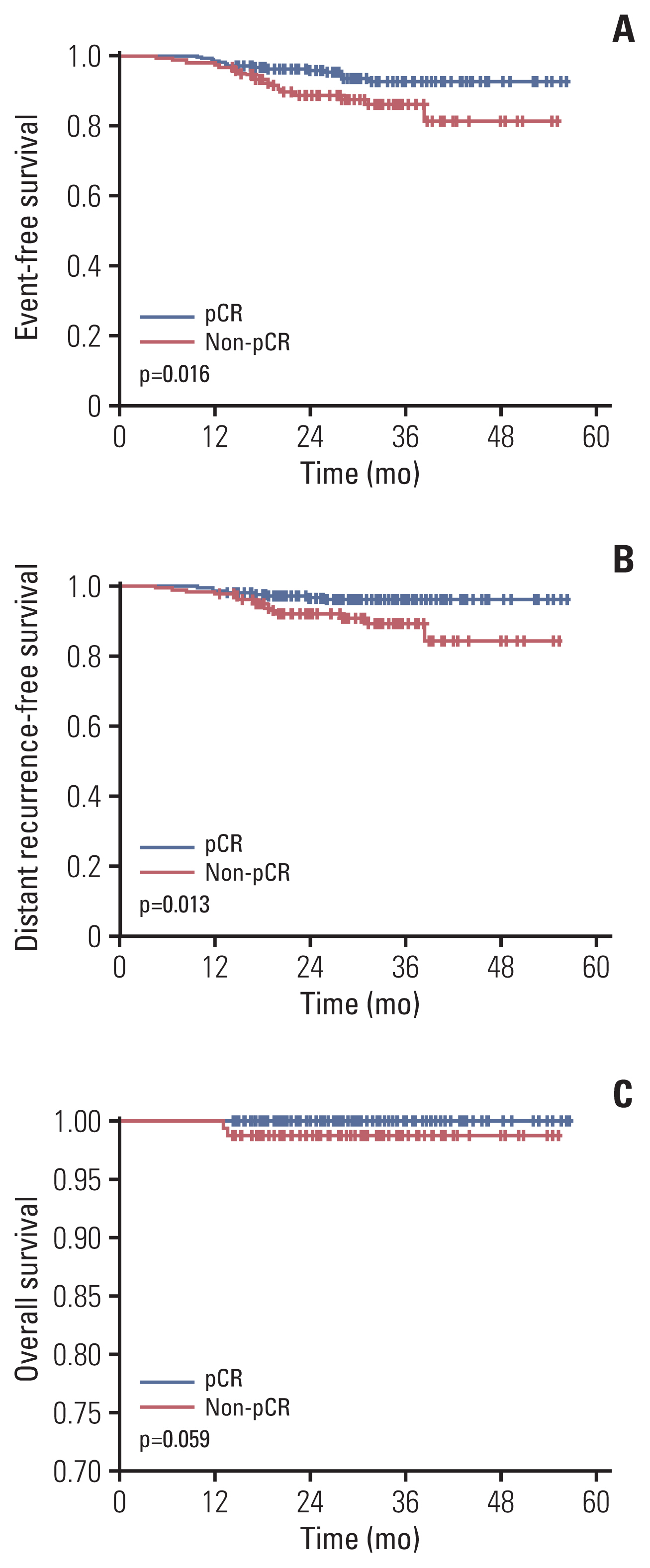
Event-free survival (A), distant recurrence-free survival (B), and overall survival (C) according to pathologic complete response (pCR) or non-pCR.
We also analyzed the association between survival rate and RCB class (S4 Fig.). EFS was associated with red blood cell (RBC) class: the 3-year EFS of RCB classes 0, 1, 2, and 3 were 93.0%, 93.3%, 82.1%, and 62.5%, respectively, p < 0.001. DRFS and OS were also associated with RCB class. Three-year DRFS of RCB classes 0, 1, 2, and 3 were 96.0%, 93.3%, 88.2%, and 72.9%, respectively (p=0.014). OS was associated with RCB class (p < 0.001).
The effects of key characteristics on EFS were shown in Fig. 2B. Clinical stage IIIB or IIIC (HR, 8.83; 95% CI, 2.58 to 30.15; p=0.001) and pCR status (HR, 0.39; 95% CI, 0.18 to 0.86; p=0.019) demonstrated effects on EFS.
4. Safety and toxicity
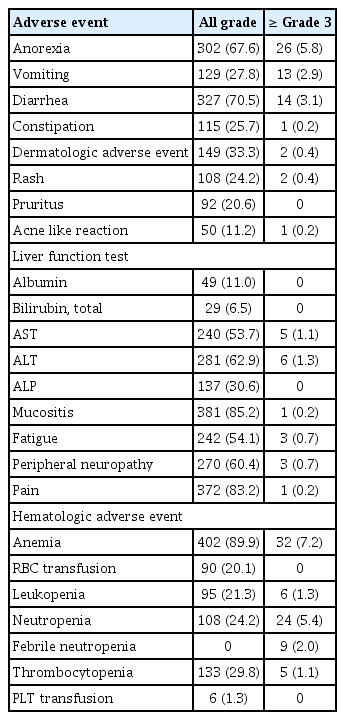
Mucositis (85.2%), pain (83.2%), and diarrhea (70.5%) were the most common non-hematologic adverse events (Table 2). In terms of grade 3 or higher adverse events, anorexia (5.8%) and diarrhea (3.1%) were most commonly observed. Hematologic adverse events were also frequently observed (Table 2). Anemia (89.9%) was the most commonly observed adverse events followed by thrombocytopenia (29.8%). Grade 3 or higher anemia occurred in 7.2% of patients and 90 patients (20.1%) received RBC transfusion. Neutropenia (24.5%) was frequently observed even though prophylactic pegfilgrastim was used at every NAC cycle. Grade 3 or higher neutropenia occurred in 5.4% and febrile neutropenia in 2.0%.
Five patients (1.1%) did not complete six cycles of neoadjuvant TCHP chemotherapy. Two patients (0.4%) only received three cycles, four cycles in two patients (0.4%) and five cycles in one patient (0.2%). RDIs of docetaxel and carboplatin were 0.965 and 0.876, respectively (S5 Table). The RDIs of both decreased as cycles progressed.
In terms of cardiac toxicity, median left ventricle ejection fraction (LVEF) at baseline echocardiography was 65.6 (interquartile range, 62 to 69) and 64.0 (interquartile range, 60 to 67). We observed ejection fraction (EF) decrease in 257 patients during NAC and more than 10% decrease of EF was observed in 88 patients. However, no patients underwent significant declines in LVEF (≥ 10% points from baseline to < 50%) and symptomatic left ventricle systolic dysfunction (S6 Fig.).
5. Treatment after curative surgery
After curative surgery, patients received adjuvant targeted agents per the established protocol. In BC patients achieving pCR, 96.5% received adjuvant trastuzumab; 3.5% received the trastuzumab and pertuzumab combination. In BC patients without pCR, adjuvant trastuzumab was used in 88.8% of patients, the trastuzumab and pertuzumab combination in 7.5%, and T-DM1 in 3.7%.
Of 447 patients, 411 (91.9%) patients received adjuvant radiotherapy (RTx) after curative surgery and 258 patients (62.8%) of patients received adjuvant RTx were performed in our institute. We evaluated the relationship between the radiation dose and pCR status. In this analysis, patients who achieved pCR received less radiation dose compared with patients without pCR (p < 0.001) (S7 Table).
Discussion
We evaluated the efficacy and safety profile of neoadjuvant TCHP chemotherapy in real world practice. pCR rate was 64% and 3-year EFS was 90.6%. In terms of adverse events (AEs), approximately 90% of patients experienced anemia. Mucositis, pain, and diarrhea were the most frequently observed non-hematologic AEs.
This result was compatible with that of the TRYPHAENA clinical trial [12]. This previous clinical trial demonstrated a pCR rate of 66% and a 3-year DFS rate of 90% [13].
We analyzed the incidence of down-staging in terms of axillary lymph node status, one of the advantages of NAC. Approximately 72.7% of patients experienced down-staging in terms of axillary node evaluation. Moreover, we performed further subgroup analysis to find the factors associated with pCR and survival. Hormone receptor negativity, low clinical stage (stage IIA–IIIA), and post-menopausal status were favorable to pCR. In terms of survival, clinical stage and pCR affected the EFS. Interestingly, hormone receptor negativity increased pCR rate but negatively affected EFS although statistical significance was marginal (p=0.055). Previous studies have suggested that hormone receptor-negative, HER2-positive BC had higher pCR rate compared with hormone receptor-positive, HER2-positive BC, whereas progression-free survival (PFS) was longer in hormone receptor-positive, HER2-positive BC rather than hormone receptor-negative, HER2-positive BC according to the NeoSphere trial.
Moreover, clinical stage significantly affected survival regardless of pCR status. This result suggests that negative hormone receptor status with initially high clinical stage BC has increased disease recurrence even though pCR had been achieved. Longer-term follow-up is warranted to confirm our suggestion.
In terms of AEs, there are s differences between our clinical experience and what was observed in clinical trial [12]. We observed anemia of 89.9% and 20.1% of patients received RBC transfusion compared with anemia of 36.8% in the clinical trial. The incidence of neutropenia (24.2%) was lower than that of the clinical trial (48.7%); however, prophylactic pegfilgrastim was administered in real world practice. We observed high incidence of all grade AEs in both non-hematologic and hematologic fields. But grade 3 or higher AEs were not frequently observed. In our data, median age of patients was 49 and 90% of patients were under 60 years of age. We suggested that young aged patients resulted low incidence of high-grade AEs.
RDI of docetaxel decreased during NAC. Especially patients over 60 years of age received less dose of docetaxel compared with whom under 60 years of age. This result suggested that elderly patient had high risk of serious AEs and dose reduction would be needed. Therefore, physicians should carefully examine elderly patients with underlying disease during NAC with TCHP regimen. In terms of carboplatin, we initially decreased the dosage of carboplatin in patients who had risk factors of renal impairment or emesis. Therefore, the dose intensity of carboplatin in the first cycle was low despite the high proportion of young patients.
The combination of docetaxel, pertuzumab, and trastuzumab is used for metastatic HER2-positive BC as first-line treatment; its efficacy was demonstrated in the CLEOPATRA clinical trial [4]. The post hoc analysis of duration of docetaxel administration showed that 14.2% of patients received fewer than six cycles because of AEs. Interestingly, these patients had shorter PFS and OS compared with those who received six cycles of docetaxel treatment [17]. Another post hoc analysis of the safety profile in Asian patients showed that these patients had more frequent docetaxel dose reduction than patients in other regions because Asian patients experienced more AEs. However, efficacy in terms of PFS and OS was similar in Asian patients to those from other regions [18].
Approximately 90% of patients in the TRYPAENA trial received the scheduled number of cycles of docetaxel and carboplatin [12]. In our study, only five (1.1%) of our patients failed to receive six cycles of treatment, and the docetaxel RDI was 0.965 and that of carboplatin was 0.876. We suggest that high RDIs of cytotoxic agents were possible due to prophylactic use of pegfilgrastim and relatively young aged patients, but high incidence of anemia and thrombocytopenia occurred because of high RDIs. In the analysis of the relationship between the RDIs of cytotoxic agents and pCR status, we did not observe the impact of RDI on pCR status. This result may be preliminary but considering toxicities of TCHP regimen, careful dose modification is necessary to maintain a balance between efficacy and safety of neoadjuvant TCHP treatment.
Although this study s retrospective analysis of neoadjuvant TCHP chemotherapy, the sample size is relatively large, and details of AEs are described. Neoadjuvant TCHP regimen is now popularly used for HER2-positive BC and therefore RWE of this regimen may be useful as treatment reference. In contrast with previous clinical trial, we focused on the factors affecting the efficacy of NAC and NAC-related AEs rather than cardiac toxicity. Relatively short follow-up duration is limitation of our study and long-term follow-up would be warranted.
In conclusion, neoadjuvant TCHP therapy had a pCR rate of 64% and a 3-year EFS of 90% in RWE. In terms of toxicity profile, anemia was frequently observed and adequate management including occasional transfusion was required.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was reviewed and approved by the Institutional Review Board (IRB) of Samsung Medical Center, Seoul, Korea (IRB No. 2019-04-021). The requirement for informed consent was waived due to the use of medical records with retrospective clinical data.
Author Contributions
Conceived and designed the analysis: Kim SW, Park YH.
Collected the data: Kim JY, Nam SJ, Lee JE, Yu J, Chae BJ, Lee SK, Ryu JM, Ahn JS, Im YH, Kim SW, Park YH.
Contributed data or analysis tools: Kim JY, Ahn JS, Im YH, Kim SW, Park YH.
Performed the analysis: Kim JY.
Wrote the paper: Kim JY, Park YH.
Conflicts of Interest
YHP reports grants from Pfizer, AstraZeneca, Novartis, Merck, Roche, and Eisai. All other authors declare no competing interests.