A Real-World Efficacy of Nab-Paclitaxel Monotherapy in Metastatic Breast Cancer
Article information
Abstract
Purpose
We aimed to assess the real-world efficacy of nab-paclitaxel in metastatic breast cancer patients.
Materials and Methods
This is a retrospective study performed in two tertiary referral hospitals in Korea. Patients with metastatic breast cancer treated with nab-paclitaxel (Abraxane) between March 2016 and March 2020 were enrolled.
Results
A total of 102 patients with metastatic breast cancer were included. Patients were heavily pre-treated with a median of four prior lines of chemotherapy (5 lines when including endocrine therapy in hormone-receptor-positive patients), and 66 patients (64.7%) were exposed to taxanes in the metastatic setting. According to St. Gallen molecular subtypes, 36 patients (35.3%) were luminal A, 28 (27.5%) were luminal B, 18 (17.7%) were human epidermal growth factor receptor 2–positive and 20 (19.6%) had triple-negative disease. Fifty patients (49.0%) were treated with a 3-weekly regimen (260 mg/m2 on day 1 every 3 weeks), and 52 (51.0%) were treated with a weekly regimen (100 mg/m2 every week). Objective response rate was 22.9%. After a median follow-up of 22.0 months, median progression-free survival (PFS) was 4.0 months (95% confidence interval [CI], 2.6 to 4.8) and median overall survival was 8.7 months (95% CI, 7.5 to 11.2). Patients treated with weekly regimen had longer PFS compared to 3-weekly regimen (5.5 vs. 2.3 months, p < 0.001). Multivariate analysis revealed the treatment regimen as an independent prognostic factor for PFS. There was no grade 3 or 4 hypersensitivity reaction.
Conclusion
This real-world data shows that nab-paclitaxel is a reasonable treatment option in heavily pre-treated and/or taxane-exposed metastatic breast cancer patients.
Introduction
Traditionally, anthracycline and taxanes are the preferred chemotherapy agents in metastatic breast cancer. Taxanes (solvent-based paclitaxel and docetaxel) induce cell-cycle arrest and apoptosis by stabilizing microtubules [1]. Paclitaxel is hydrophobic and therefore requires a solvent such as polyethoxylated castor oil (Cremophor EL). Solvents included in the solvent-based paclitaxel increase the risk of acute hypersensitivity reaction and peripheral neuropathy [2]. As such, corticosteroid premedication is required in solvent-based paclitaxel to prevent hypersensitivity reaction. Nanoparticle albumin-bound (nab)-paclitaxel (Abraxane) is a solvent-free form of paclitaxel developed to overcome shortcomings from the solvent-based form. Because of the albumin-bound form, nab-paclitaxel could deliver drugs to the target sites more effectively without forming redundant micelles. More importantly, it does not require corticosteroid premedication. Compared to solvent-based paclitaxel, previous studies demonstrated comparable or better efficacy of nab-paclitaxel with favorable safety profiles [3,4].
Recently, immune checkpoint inhibitors have shown promising results in metastatic breast cancer. In the IMpassion130 trial, atezolizumab plus nab-paclitaxel was superior to nab-paclitaxel monotherapy in metastatic triple-negative breast cancer [5]. Nab-paclitaxel was selected as a partner drug of the atezolizumab as premedication of corticosteroid is not required. There are concerns that corticosteroid may have a negative effect on drug efficacy of immune checkpoint inhibitors [6,7].
While nab-paclitaxel showed promising results in metastatic breast cancer patients, most studies were performed in earlier lines of treatment [3,4,8]. In addition, there is a paucity of data in the real-world setting, especially in the Asian population. We conducted this study to assess the real-world efficacy of nab-paclitaxel monotherapy in Korean breast cancer patients.
Materials and Methods
1. Study design, patients, and data collection
This is a retrospective study of metastatic breast cancer patients treated with nab-paclitaxel monotherapy. Patients meeting the inclusion and exclusion criteria were enrolled from two tertiary referral hospitals in South Korea between March 2016 and March 2020 (Seoul National University Hospital [SNUH] and Seoul National University Bundang Hospital [SNUBH]).
Eligible patients were women aged 20 years or older at diagnosis with confirmed breast cancer pathology. Patients who received nab-paclitaxel monotherapy in a metastatic setting were included. Patients treated with combination therapy of nab-paclitaxel with other chemotherapy agents or who had double primary cancer were excluded. Eligible patients were identified from the electronic database of SNUH and SNUBH, and medical charts were reviewed using the electronic medical record system of each institute.
Patients received nab-paclitaxel as a weekly regimen (100 mg/m2 every week) or a 3-weekly regimen (260 mg/m2 on day 1 every 3 weeks, once every 3 weeks) on the discretion of the treating physician. Based on performance status and toxicities, dose reduction or interruptions were made by the treating physicians.
2. Analysis of tumor subtype
Immunohistochemical staining was performed with formalin-fixed paraffin-embedded tissue at initial diagnosis or at the time of recurrence with metastatic disease. Nuclear expression of tumor cells was interpreted as positive for estrogen receptor (ER) and progesterone receptor (PR), while membrane staining of tumor cells was considered positive for human epidermal growth factor receptor 2 (HER2). The expression of ER and PR was positive when ≥ 1% of the tumor cells were stained according to the 2010 ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guidelines [9]. The HER2 positivity was assessed based on the 2013 ASCO/CAP guidelines [10]. Patients were categorized as either ‘luminal A,’ ‘luminal B,’ ‘HER2-positive,’ or ‘triple-negative’ according to the 2011 St. Gallen Consensus Panel [11].
3. Statistical analysis
The objective of this study was to reveal the real-world efficacy of nab-paclitaxel in terms of objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety. The best tumor response was evaluated by computed tomography scans using Response Evaluation Criteria in Solid Tumors guideline ver. 1.1. ORR was defined as combined proportions of patients with the best response of complete response (CR) and partial response (PR). DCR was defined as a combined proportion of CR, PR, and stable disease (SD) for 12 weeks or longer. PFS was calculated from the date of chemotherapy initiation to disease progression or death, whichever occurred first. Data from patients who were free of disease progression or loss to follow-up were censored at the date of the last follow-up visit. Patients who discontinued nab-paclitaxel due to its toxicity were censored at the time of the last dose injection. OS is the time from the date of chemotherapy initiation to death from any cause. In the current study, we defined patients treated with four or more previous chemotherapy lines as heavily treated. Primary taxane resistance was defined when the best response to prior taxane was a progressive disease (PD). Taxane sensitive relapse was defined in patients who had objective response to previous neoadjuvant taxane or those who had disease relapse after 6 months of adjuvant taxane treatment. Categorical variables were compared using the chi-square test. PFS and OS were calculated with the Kaplan-Meier method, and comparisons were made using the log-rank tests. Hazard ratios (HR) were calculated using the multivariate Cox proportional hazard model. Toxicity was evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. We considered a p-value of less than 0.05 to be statistically significant. Statistical analysis was performed using STATA version 16.0 (StataCorp LP, College Station, TX).
Results
1. Baseline characteristics
A total of 102 patients were treated with nab-paclitaxel monotherapy between March 2016 and March 2020 (data cutoff, 31 October 2020). The baseline characteristics are summarized in Table 1. According to subtypes of breast cancer by 2011 St. Gallen consensus, 36 patients (35.3%) had luminal A, 28 (27.5%) had luminal B, 18 (17.7%) had HER2-positive, and 20 (19.6%) had triple-negative disease. Patients were treated with a median of four lines of previous chemotherapy in the metastatic setting (5 lines when including endocrine therapy in hormone-receptor–positive patients). Nine patients received nab-paclitaxel in the 1st line, 13 in the 2nd line, 11 in the 3rd line, and 54 patients in the 5th or later lines of cytotoxic chemotherapy. Among patients with luminal A or B subtypes, 84.3% failed at least one line of endocrine therapy, and 94.4% of HER2-positive patients had failed at least one line of HER2-directed therapy before initiation of nab-paclitaxel. Sixty-six patients (64.7%) were previously exposed to taxanes in the metastatic setting, and 54 patients (52.9%) received four or more lines of chemotherapy in the metastatic setting. Fifty patients (49.0%) were treated with a 3-weekly regimen (260 mg/m2 once every 3 weeks), and 52 patients (51.0%) with a weekly regimen (100 mg/m2 once weekly). The mean actual dose intensities (±standard deviation) of nab-paclitaxel were 76.62 (±10.18) mg/m2 per week in the 3-weekly and 67.95 (±15.67) mg/m2 per week in the weekly regimen.
2. Treatment outcomes
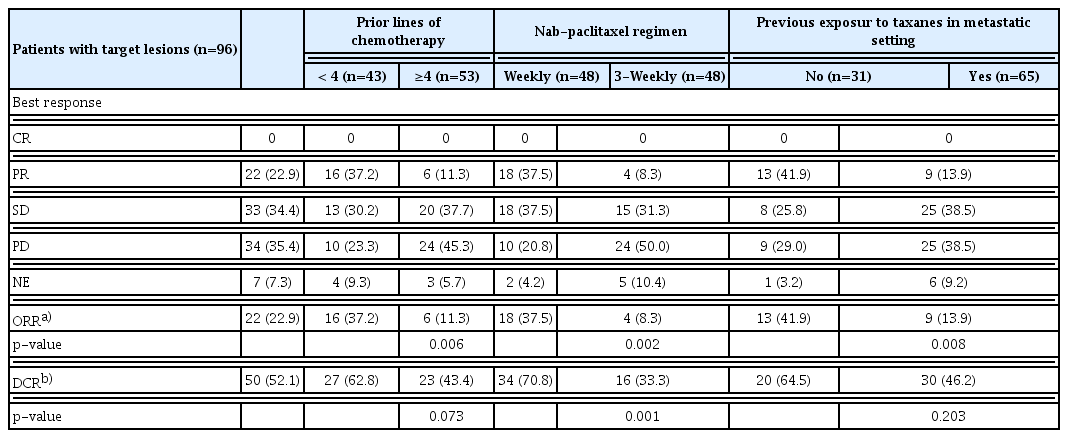
Among a total of 102 patients, 96 patients (94.1%) had measurable lesions. Of 96 patients, there was no CR, 22 had PR (22.9%), 33 had SD (34.4%), and 34 had PD (35.4%) with an ORR of 22.9% and DCR of 52.1%. The ORR was influenced by the drug administration schedule, prior lines of chemotherapy, prior taxane exposure, and tumor subtypes. Patients treated with a weekly regimen had higher ORR than those treated with a 3-weekly regimen (37.5% vs. 8.3%, p=0.002). The ORR was 11.3% in heavily treated patients (≥ 4 lines of previous chemotherapy) compared to 37.2% in the less treated (p=0.002). Taxane un-exposed patients had higher ORR compared to patients who received taxanes in the metastatic setting (41.9% vs. 13.9%, p=0.004) (Table 2). Patients with luminal A or HER2-positive disease had higher ORR compared to patients with luminal B or triple-negative disease (38.2% in luminal A, 11.1% in luminal B, 29.4% in HER2-positive, and 5.6% in the triple-negative; p=0.024).
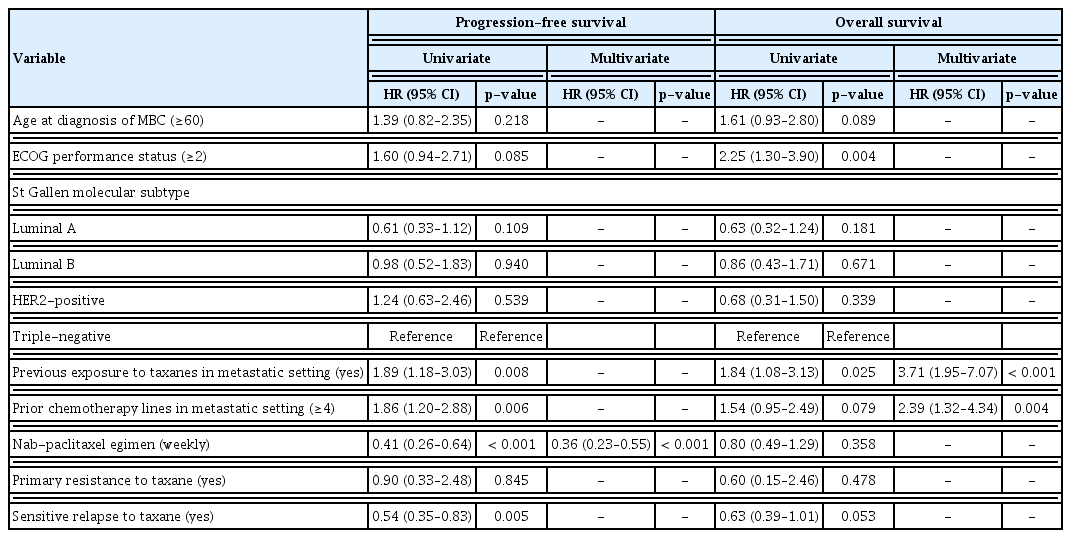
After a median follow-up duration of 22.0 months (range, 7.5 to 55.6), the estimated median PFS was 4.0 months (95% confidence interval [CI], 2.6 to 4.8), and OS was 8.7 months (95% CI, 7.5 to 11.2) (Fig. 1). Patients who received a weekly regimen had longer PFS than patients treated with a 3-weekly regimen (5.5 months vs. 2.3 months, p < 0.001). However, there was no significant difference in OS between the two groups (7.8 months vs. 9.3 months, p=0.356) (Fig. 2). PFS was also influenced by the number of previous chemotherapies and prior exposure to taxanes in the metastatic setting. Heavily treated patients had worse PFS than less treated patients (2.5 months vs. 5.3 months, p=0.005). PFS was 3.1 months in taxane-exposed patients compared to 5.5 months in taxane naïve patients in the metastatic setting (p=0.007). There was no statistical difference in PFS according to molecular subtypes. To adjust for the baseline characteristics, Cox proportional hazard analysis was performed in a forward stepwise method. Covariates included in the Cox proportional hazard analysis were age, Eastern Cooperative Oncology Group (ECOG) performance status score (0–1 vs. 2–3), tumor subtype, nab-paclitaxel regimen (weekly vs. 3-weekly), prior taxane exposure, prior lines of chemotherapy (< 4 vs. ≥ 4), and primary resistance or sensitive relapse after conventional taxanes. Multivariate analysis revealed nab-paclitaxel regimen as an independent prognostic factor for PFS. Patients treated with weekly regimen had improved PFS compared to those treated with 3-weekly regimen (adjusted HR, 0.36; 95% CI, 0.23 to 0.55; p < 0.001) (Table 3). Prior taxane exposure in the metastatic setting, primary taxane resistance, sensitive relapse, prior lines of chemotherapy, and tumor subtypes were not associated with PFS in the multivariate analysis. In the aspect of OS, prior exposure to taxanes in the metastatic setting (adjusted HR, 3.71; 95% CI, 1.95 to 7.07; p < 0.001) and more than four lines of previous chemotherapy (adjusted HR, 2.39; 95% CI, 1.32 to 4.34; p=0.004) were an independent negative prognostic factor (Table 3).
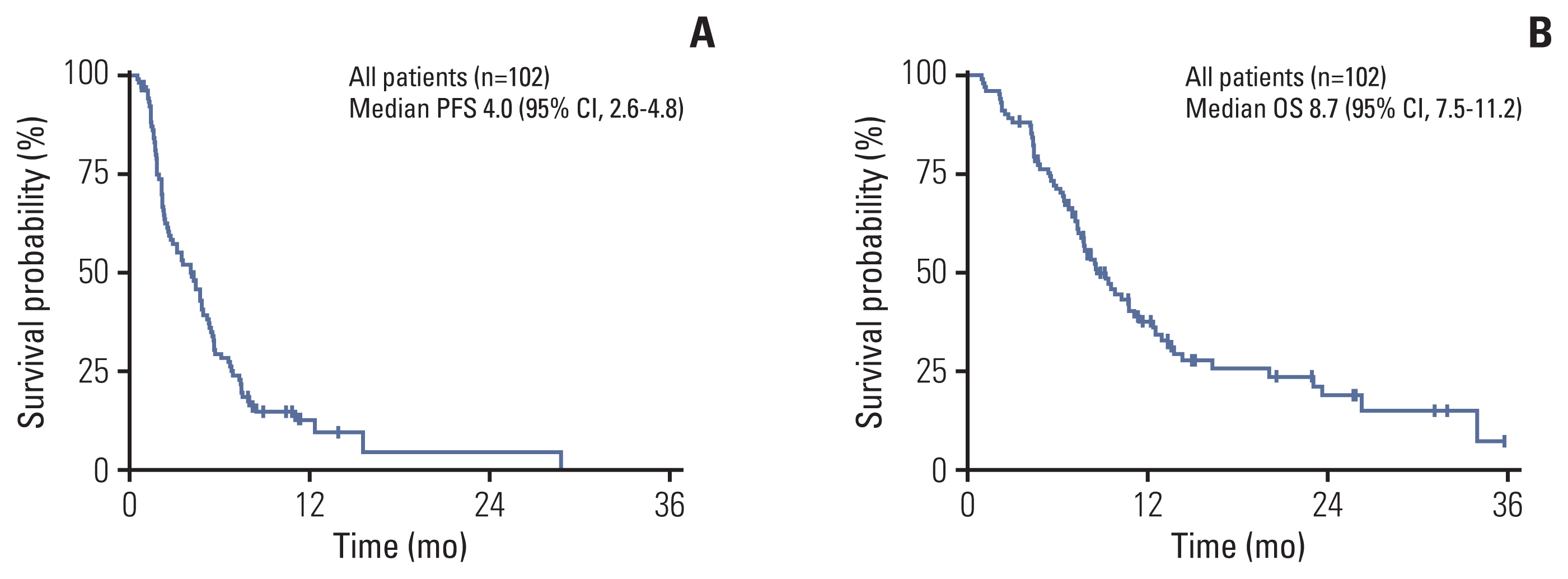
Kaplan-Meier curves of progression-free survival (PFS, A) and overall survival (OS, B) in metastatic breast cancer treated with nab-paclitaxel. CI, confidence interval.
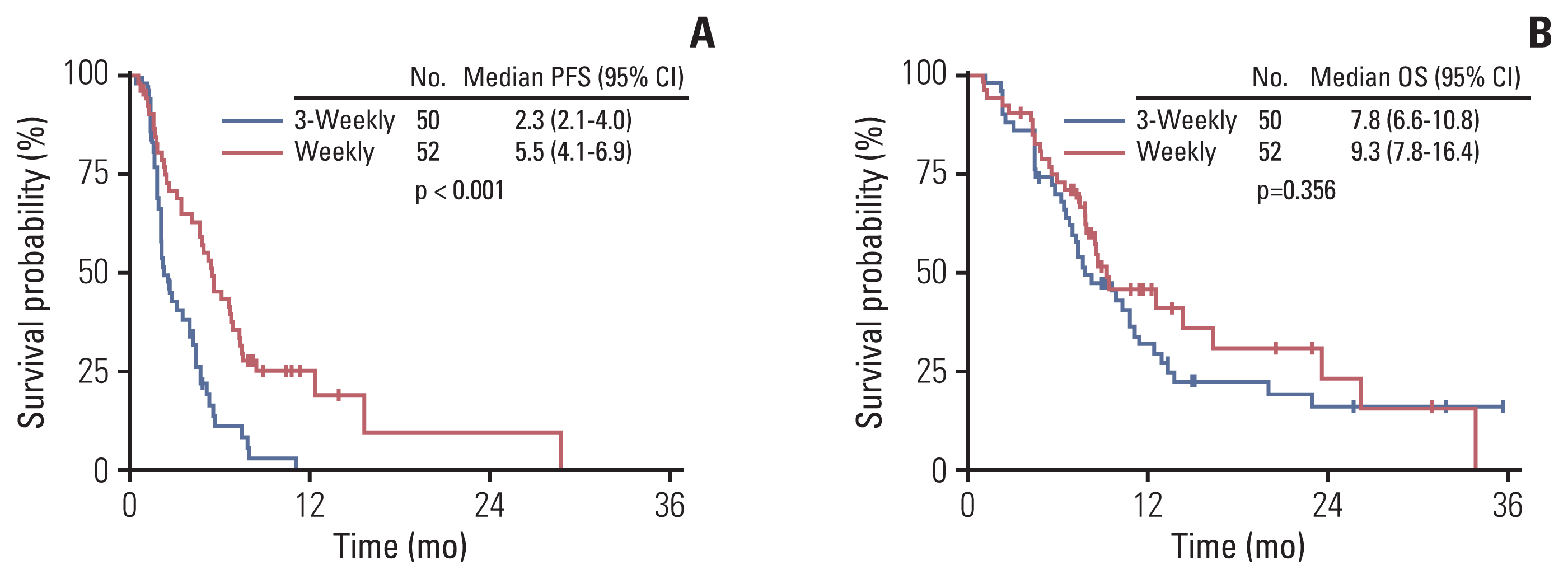
Kaplan-Meier curves of progression-free survival (PFS, A) and overall survival (OS, B) by dosing schedule of nab-paclitaxel. CI, confidence interval.
3. Safety
According to CTCAE ver. 4.0, 13.7% of patients (14/102) experienced grade 3/4 peripheral neuropathy, leading to cessation in 4.9% of patients (5/102). Grade 3/4 neutropenia and thrombocytopenia occurred in 26.5% (27/102) and 3.9% (4/102) of patients, respectively. Although 1.9% (2/102) of patients had a grade 1/2 hypersensitivity reaction, no patients had a grade 3/4 hypersensitivity reaction despite the absence of corticosteroid premedication.
As for the administration schedule, there was higher incidence of grade 3/4 neutropenia (40.4% vs. 12.0%, p=0.001) and tendency of higher incidence of grade 3/4 peripheral neuropathy (20.0% vs. 7.7%, p=0.071) in patients treated with weekly regimen compared to those treated with 3-weekly regimen. Relative dose intensity was lower in weekly regimen compared to 3-weekly regimen (68.0% vs. 88.4%, p > 0.001) due to frequent dose modification. However, toxicity was generally manageable after dose reduction and treatment-related adverse events leading to treatment discontinuation was same for both arm (3 patients for each group). Three patients treated with 3-weekly regimen and two patients treated with weekly regimen discontinued treatment due to severe peripheral neuropathy. Additional one patient in the weekly regimen discontinued treatment due to paclitaxel-related maculopathy. Treatment-related death occurred in one patient treated with weekly regimen owing to neutropenic sepsis.
Discussion
This study investigated the real-world efficacy of nab-paclitaxel in Korean women with metastatic breast cancer. The real-world efficacy and safety of nab-paclitaxel were comparable to those of previously conducted clinical trials. Our retrospective study results show that nab-paclitaxel is a promising treatment option for heavily pre-treated or taxane-exposed metastatic breast cancer patients.
The principal treatment strategy for metastatic breast cancer is systemic therapy (including cytotoxic chemotherapy, endocrine therapy, and HER2-directed therapy) depending on its tumor subtype. Anthracycline and taxane are the preferred cytotoxic agents. Paclitaxel is hydrophobic and requires a solvent to be used as a therapeutic agent. However, the solvent included in paclitaxel increases acute hypersensitivity reaction and reduces drug delivery by forming micelles in the plasma. In addition, this entrapment by the solvent may hinder clearance and prolong exposure of paclitaxel in the circulation, resulting in increased systemic toxicities such as neutropenia. Compared to solvent-based paclitaxel, nab-paclitaxel has benefits of enhanced permeability, retention effect, and natural albumin transcytosis pathway, which improves drug distribution [12–15]. Also, nab-paclitaxel does not require corticosteroid premedication. The NCCN guideline recommends nab-paclitaxel as a substitute for paclitaxel or docetaxel, especially in the medical necessity of corticosteroid avoidance (hypersensitivity reaction, uncontrolled diabetes mellitus) [16].
Several trials have validated the clinical efficacy and safety of nab-paclitaxel in metastatic breast cancer. In a pivotal phase III trial, nab-paclitaxel (260 mg/m2, once every 3 weeks) showed prolonged PFS compared to standard solvent paclitaxel (175 mg/m2, once every 3 weeks) (median, 23.0 vs. 16.9 weeks; p=0.006) with no high-grade hypersensitivity reactions despite the absence of corticosteroid premedication [4]. In a single-arm phase II trial, nab-paclitaxel (300 mg/m2, once every 3 weeks) resulted in an ORR of 48% for all patients and 64% in chemotherapy-naïve patients [8]. In another phase II trial performed in heavily pre-treated patients, weekly administration (weekly for the first 3 of 4 weeks) of 100 mg/m2 or 125 mg/m2 of nab-paclitaxel demonstrated ORR of 14% and 16%, respectively [17]. Lastly, in the phase II trials performed in chemotherapy-naïve patients, weekly nab-paclitaxel (150 mg/m2, weekly for the first 3 of 4 weeks) demonstrated significantly longer PFS (12.9 months vs. 7.5 months, p=0.0065) and higher ORR (49% vs. 35%) compared to docetaxel (100 mg/m2, once every 3 weeks) [3]. Previously conducted clinical trials of nab-paclitaxel included patients in relatively earlier lines. Two studies were performed in the first-line setting [3,5], and more than 90% of patients received nab-paclitaxel within the third line of therapy in the other three trials [4,8,17]. There is a paucity of data on the efficacy of nab-paclitaxel in heavily pre-treated patients. Notably, in the current study, the median line of previous chemotherapy was 4, and 52.9% of patients received nab-paclitaxel in the 5th or later lines of systemic chemotherapy. There was still a modest benefit in heavily pre-treated patients with an ORR of 11.3%, median PFS of 2.5 months, median OS of 7.7 months, and 1-year OS rate of 14.8%.
Recently, immune checkpoint inhibitors have shown promising results in triple-negative breast cancer patients. As there are concerns that corticosteroid may have a negative effect on the efficacy of immune checkpoint inhibitors, nab-paclitaxel has been an attractive partner for various immune checkpoint inhibitors. In the pivotal IMpassion130 study, atezolizumab combined with nab-paclitaxel showed improved PFS compared to nab-paclitaxel monotherapy (7.5 months vs. 5.0 months, p < 0.001) [5]. On the other hand, IMpassion131 study failed to show a clinical benefit of atezolizumab when combined with paclitaxel in the first-line setting of triple-negative disease [18]. There are critics that corticosteroids may have blunted the effect of atezolizumab in the IMpassion131 study. As nab-paclitaxel is highlighted as a potential partner for various immune checkpoint inhibitors [19], the indication of nab-paclitaxel is to be expanded.
There is evidence that the treatment schedule may affect the treatment outcome of solvent-based paclitaxel. Weekly regimen of paclitaxel showed superior outcome compared to 3-weekly regimen in metastatic breast cancer in terms of ORR (42% vs. 29%, p=0.0004) and time-to-progression (9 months vs. 5 months, p < 0.0001) [20]. In addition, weekly paclitaxel regimen showed better efficacy in adjuvant setting, concerning 5-year disease-free survival (81.5% vs. 76.9%; odds ratio, 1.27; p=0.006) and 5-year OS (89.7% vs. 86.5%; odds ratio, 1.32; p=0.01) compared to 3-weekly paclitaxel regimen [21]. However, there is no report on whether there is a difference in nab-paclitaxel efficacy according to the treatment schedule. In one real-world data including 697 patients, there was no difference in the clinical efficacy of nab-paclitaxel regarding the treatment schedule. However, 40.2% of patients in this cohort received nab-paclitaxel as first-line therapy, and only 15.4% received nab-paclitaxel as 4th or later lines of treatment [22]. Currently, nab-paclitaxel is accepted in both weekly or 3-weekly regimens [23]. In the present study, patients treated with a weekly regimen showed longer PFS and higher ORR compared to those treated with a 3-weekly regimen. While previous taxane exposure and taxane resistance did not affect PFS, multivariate analysis revealed a weekly regimen as a preferable regimen compared to a 3-weekly regimen in terms of PFS (adjusted HR, 0.29; p < 0.001). It is generally accepted that the weekly regimen has an advantage in dose modification, delays, and managing toxicity compared to the 3-weekly regimen. We believe a weekly nab-paclitaxel regimen could be a reasonable option, especially in heavily treated patients and even those who experienced relapse after neoadjuvant/adjuvant conventional taxanes.
The decision for choosing a schedule was on the discretion of the treating physician. In general, a physician may prefer the weekly regimen for patients with poor performance status or those who experienced severe toxicity from the previous chemotherapy, as it allows close patient monitoring. In this retrospective analysis, although statistically not significant, there was the tendency of a higher proportion of patients with ECOG performance status 2–3 in weekly regimen compared to the 3-weekly regimen (23.1% vs. 16.0%, p=0.368) (Table 1). These factors may have also affected the higher incidence of toxicity in patients treated with the weekly regimen compared to those treated with the 3-weekly regimen. Subsequently, more patients treated with the weekly regimen (63.5%, 33/52) underwent dose modifications than the 3-weekly regimen (54.0%, 27/50), but a lower proportion of patients discontinued treatment in the weekly (5.8%, 3/52 vs. 6.0%, 3/50). Altogether, the weekly regimen is tolerable and elicits superior outcomes compared to the 3-weekly regimen.
There are some limitations in the present study. First, many patients were heavily treated with inconsistent previous exposure to chemotherapeutic agents, and the criteria for dose modification were different between physicians. Second, we could not thoroughly evaluate toxicity such as grading of hypersensitivity reaction and peripheral neuropathy related to patients’ quality of life.
In conclusion, this real-world data shows that nab-paclitaxel is an effective treatment option in metastatic breast cancer. Nab-paclitaxel was well tolerated and showed clinical benefit even in heavily pre-treated or taxane-exposed patients. A weekly regimen may be more beneficial than a 3-weekly in heavily pre-treated patients.
Notes
Ethical Statement
This study was reviewed and approved by the institutional review board (IRB) of SNUH (No. 1904-026-1024) and SNUBH (No. B-2004/608-401). This study was conducted in accordance with the Principles of the Declaration of Helsinki. Requirement of informed consent was waived by the IRB for this retrospective analysis. Data were anonymized and de-identified before analysis.
Author Contributions
Conceived and designed the analysis: Lee DW, Im SA.
Collected the data: Kim JS, Suh KJ, Lee DW.
Contributed data or analysis tools: Kim JS, Suh KJ, Lee DW, Woo Gu, Kim M, Kim SH, Ryu HS, Lee KH, Kim TY, Han SW, Park SY, Park IA, Kim JH, Im SA.
Performed the analysis: Kim JS, Suh KJ, Lee DW, Im SA.
Wrote the paper: Kim JS, Suh KJ, Lee DW, Woo Gu, Kim M, Kim SH, Ryu HS, Lee KH, Kim TY, Han SW, Park SY, Park IA, Kim JH, Im SA.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This research was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HA17C0055 and 1720150).