Physical and Biological Characteristics of Particle Therapy for Oncologists
Article information
Abstract
Particle therapy is a promising and evolving modality of radiotherapy that can be used to treat tumors that are radioresistant to conventional photon beam radiotherapy. It has unique biological and physical advantages compared with conventional radiotherapy. The characteristic feature of particle therapy is the “Bragg peak,” a steep and localized peak of dose, that enables precise delivery of the radiation dose to the tumor while effectively sparing normal organs. Especially, the charged particles (e.g., proton, helium, carbon) cause a high rate of energy loss along the track, thereby leading to high biological effectiveness, which makes particle therapy attractive. Using this property, the particle beam induces more severe DNA double-strand breaks than the photon beam, which is less influenced by the oxygen level. This review describes the general biological and physical aspects of particle therapy for oncologists, including non-radiation oncologists and beginners in the field.
Introduction
Radiotherapy has long been in clinical use for cancer treatment. The DNA of tumors and healthy cells is injured by ionizing radiation, resulting in complex biochemical reactions, prolonged abnormal cell function, and, eventually, cellular death. Beams of ionizing photons such as X-rays or gamma-rays have been used for treating various types of cancer. Currently, the widely available X-ray beam therapy (XRT) is considered as the “conventional” radiation treatment method in clinical practice.
Charged particle beam therapy (i.e., particle therapy) has been emerging clinically as a branch of radiotherapy from the late twentieth century [1]. The initial clinical implementations were conducted at the University of Tsukuba and Loma Linda University, which started clinical centers for proton therapy in 1983 and 1990, respectively [2,3] and the National Institute of Radiological Sciences in Japan, which treated patients with the carbon ion therapy in 1994 [4]. Particle therapy with protons and heavier charged particles has significant physical and biological advantages over conventional therapy [5], thus allowing them to potentially achieve more effective tumor control while sparing the surrounding normal tissues.
Proton therapy is being used worldwide, including in two centers in Korea, but carbon ion therapy is available only in a few countries, namely, Germany, Italy, Austria, Japan, and China [6]. Carbon ion therapy facilities in Korea are also under construction in two centers [7], and we expect that particle therapy will be of more use to many cancer patients in the near future. However, non-radiological oncologists or even trainees in radiation oncology are unfamiliar with particle therapy, compared with conventional radiotherapy. In this paper, we introduce the basics of physical and biological characteristics of particle therapy for oncologists and focus on some recent issues, especially proton and carbon ion therapies.
Definition and Clinical Aspects of Particle Therapy
1. Definition of particle therapy
Particle therapy for cancer treatment is a form of external beam radiotherapy using protons, neutrons, or other heavier ions (e.g., helium or carbon ions). The type of a specific particle therapy is generally based on the particles to generate beams for therapy.
2. Particle therapy in clinics
Physically, particle beams yield the benefit of precise dose localization, compared with X-rays. Particle beams deposit sharply increased energy at the last part and a very small dose in the tissue over the beam. This results in a decreased radiation dose delivered to normal tissues, compared to that in XRT, at the entry site of the radiation field and beyond the target area. For these reasons, radiation oncologists would expect radiation-induced morbidity from normal tissue damage to be smaller. It might be possible to deliver higher ablative doses of charged particles to the tumor area while reducing damage to the normal tissue. This property is particularly attractive for inoperable case or tumors adjacent to critical structures. Recently, some clinical studies reported that proton therapy might be beneficial not only for tumors in adjacent organs but also to counteract the systemic complications from radiotherapy. Less low-dose exposure during proton therapy might affect the level of lymphocytes, which are regarded as a marker of immunity and therapeutic responses [8]. In a phase II randomized study, proton therapy reduced the rate of severe radiation-induced lymphopenia in glioblastoma patients from 39% to 14%, compared with XRT [9]. Additionally, retrospective studies have supported this result [10]. Particle therapy has the potential to reduce another complication, secondary cancer. A recent study of 450,000 patients conducted using the Chinese national database reported that the risk of secondary cancer during proton therapy had an odds ratio of 0.31 compared with that in intensity-modulated radiotherapy [11]. In a Japanese study, the risk of secondary malignancy after carbon ion therapy for prostate cancer was compared to that after photon radiotherapy or surgery alone [12]. The results revealed that carbon ion therapy conferred significantly lower risk of developing secondary malignancy, with a hazard ratio of 0.80, than photon radiotherapy. The risk of secondary malignancy in carbon ion therapy was also not increased compared to that of surgery alone. Clinical studies for particle therapy have been gradually increasing, mostly based on proton therapy, or carbon ion therapy. Although there were fewer studies on other heavy particle therapies, centers using helium ion therapies have been showing good results [13,14].
Physics in Particle Therapy
1. Physical characteristics of particle therapy
Physically, charged particle beams deposit energy along their paths when traveling in the body and exhibit a unique depth-dose distribution, termed the “Bragg peak” (Fig. 1). This special physical characteristic distinguishes particle therapy from X-ray. The particles deposit most of their energy in the final millimeters of their trajectory as they slow down. This results in a steep and localized peak of dose. Photon beams or X-rays do not have a Bragg peak and, thus, deliver the maximum dose to the tissues upon entry, following which, they are gradually attenuated as they pass through the body. Nevertheless, a substantial dose is still delivered deep inside the body. This is because X-rays are a form of electromagnetic radiation that has no mass or charge; therefore, they easily pass through the body and deposit energy along the whole length of their path. Due to the nature of X-rays, multi-focus beams such as intensity-modulated radiotherapy are usually needed to irradiate deep-seated tumors with external beam X-rays with useful conformity. Because of the multi-focus beams, normal tissues around the target receive low doses of radiation. Consequently, compared with intensity-modulated radiotherapy, particle therapy can produce steeper dose gradients and a more conformal dose distribution without increasing the dose delivered to the normal tissue, using a smaller number of beams, as shown in Fig. 2.
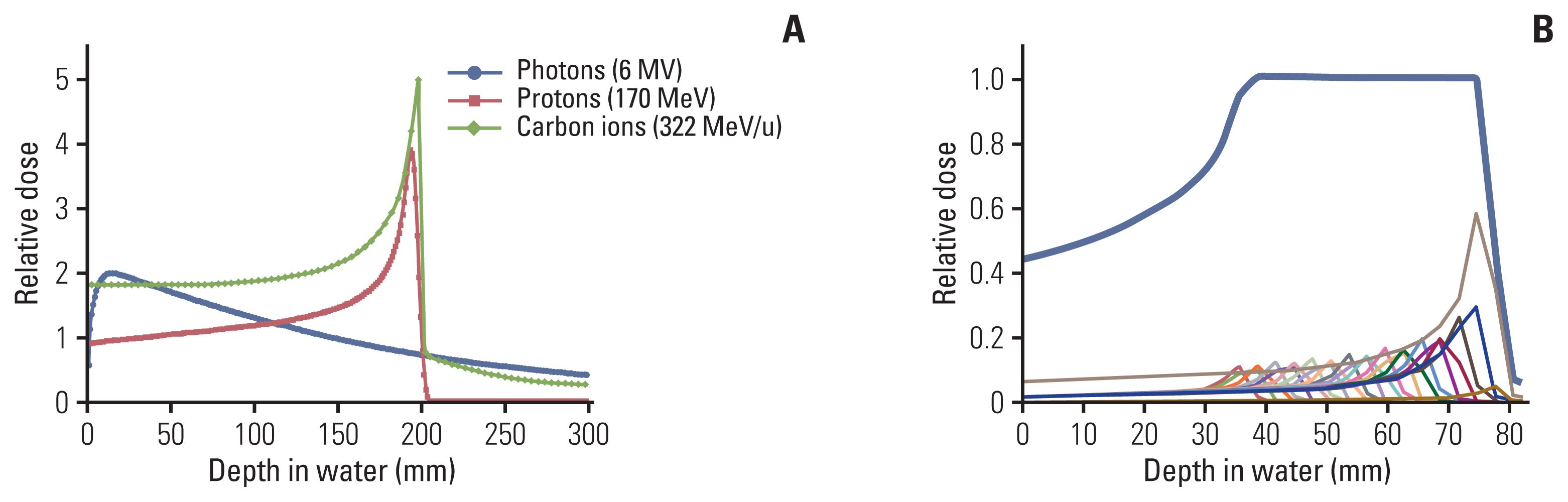
(A) Depth-dose distributions for photons, protons, and carbon ions. (B) A spread-out Bragg peak of a carbon ion beam (bold line) for a single-entry port.
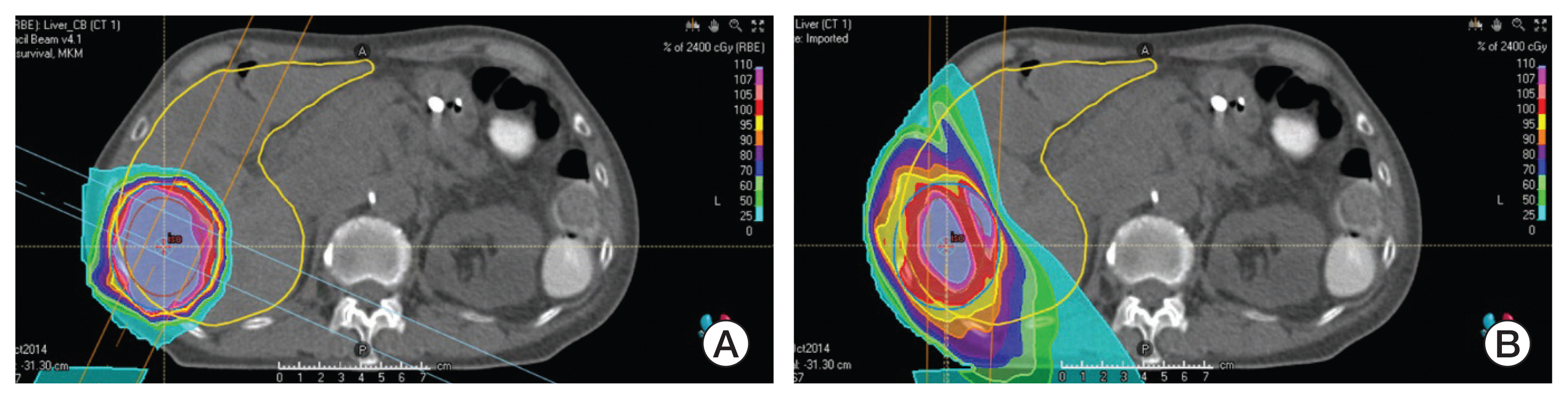
Screenshot of plan comparison between carbon ion therapy (A) and conventional X-ray intensity-modulated radiotherapy (B). Note that carbon ion beams can produce steeper dose gradients and a more conformal dose distribution without increasing the dose delivered to the normal tissue, with a smaller number of beams.
As shown in Fig. 1, the Bragg peak used in particle therapy is too sharper and thinner than that used in conventional therapy. To utilize the particle beams in radiation therapy, it is necessary to achieve broadening of the beam, termed the “spread-out Bragg Peak (SOBP),” to extend the uniform dose region to treat tumors of different depths. SOBP is the sum of several individual Bragg peaks at staggered depths.
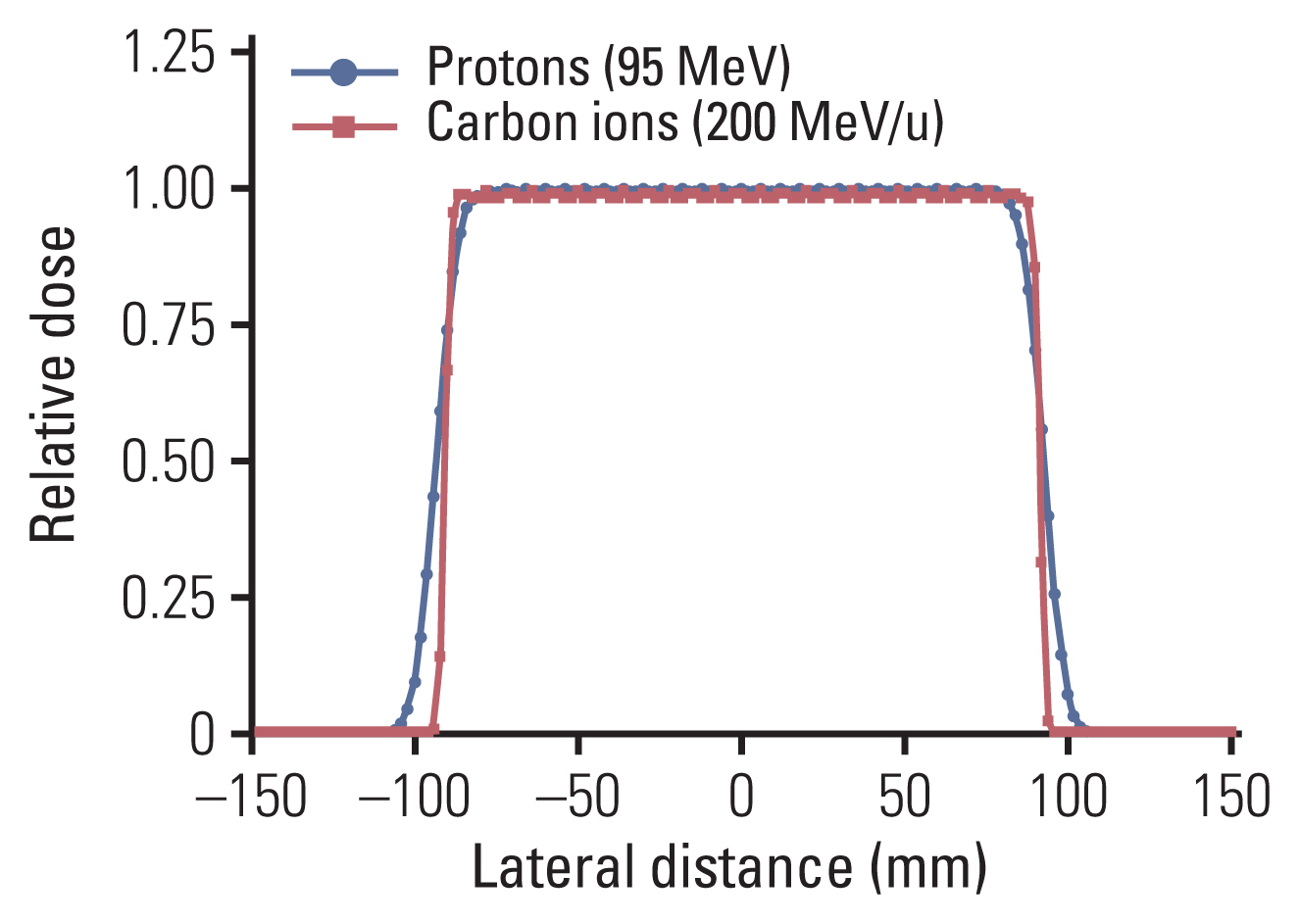
The physical characteristics vary according to the type of particle. From the point of view of dose distribution, the beam quality depends on its energy spread, range straggling, and lateral sharpness, all being smaller in magnitude with increasing particle mass. For example, carbon ion beam shows a higher physical dose concentration with a narrow penumbra compared to proton beam, as shown in Fig. 3. Another difference between heavier ions and protons is the fragmentation mechanism. Compared with that of protons, fragmentation of carbon ions (e.g., boron, beryllium, lithium, and helium) occurs because of nuclear interactions between the atoms of the irradiated tissue. The energy of the fragmentation is deposited beyond the range of the carbon ions in the so-called tail region (Fig. 1). The biological effect of this fragmentation is small because the tail contains only fragments with a low atomic number; nevertheless, this tail region of carbon ion beams should be checked through the radiation planning system if organs at risk surround the target.
2. Beam delivery systems for particle therapy
Accelerators are one of the major devices used in radiotherapy that produce and shape an electric field to accelerate charged particles. In conventional XRT, a single-pass-type accelerator (i.e., linear accelerator) is generally used to accelerate electron beams through the linear path. Unfortunately, due to the heavier mass of charged particles compared to that of electrons, the small size of the linear accelerator cannot produce a sufficient electric field for particle therapy. At present, the available option for heavier particle therapy involves efficient reuse of the electric field using circular (or multi-pass-type) accelerators (e.g., cyclotrons, synchrotrons, synchrocyclotrons) instead of a linear accelerator to reach the required energy for clinical use of particle beams.
The first particle accelerator was built in the early 1950s as a synchrocyclotron [15,16]. The first patient was treated in 1957 using proton beams at the Lawrence Berkeley National Laboratory [17–19]. As of December 2020, 57 cyclotrons, 41 synchrotrons, and 13 synchrocyclotrons have been installed in various particle therapy centers for clinical use (e.g., 250 MeV for protons and 440 MeV/u for carbon ions) [20]. In general, these circular accelerators require large space for installation compared to conventional linear accelerators. Nevertheless, during the treatment process, the time the accelerator operates is much shorter than the time for patient setup or beam alignment in the treatment room. Consequently, most institutes operate several treatment rooms per accelerator to save time and optimize treatment schedules.
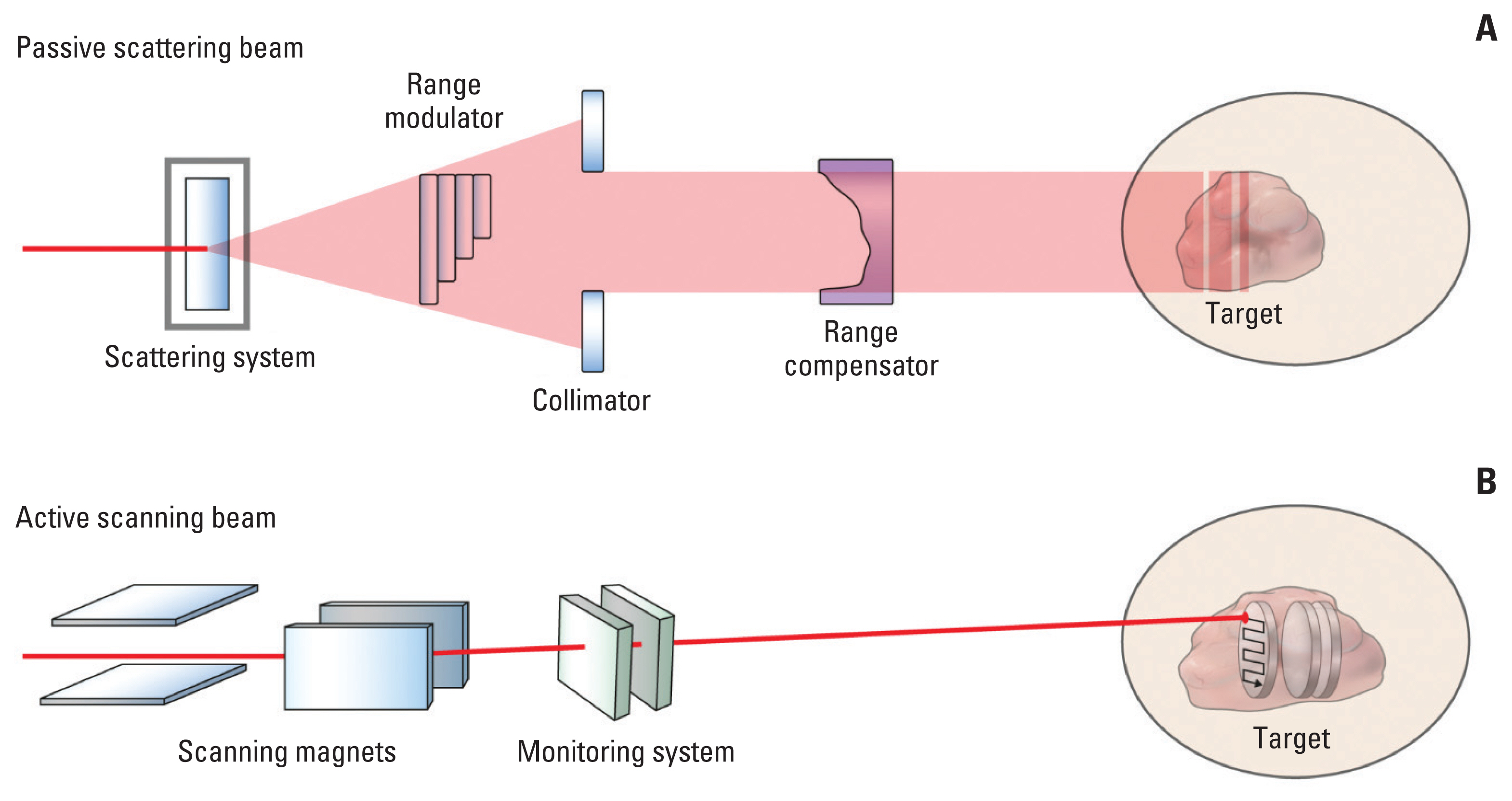
Particle therapy can be delivered using two delivery systems: (1) a beam scattering method using a passive system and (2) a beam scanning method using an active system. In the passive system, the narrow peaks are swept over a wide area by a peak filter to create an SOBP, corresponding to the target volume size. This method simultaneously uses a band modulator, a collimator, and a compensator. In the active system, the peak position is moved within the target by varying the beam energy in the accelerator or by changing the beam’s penetration using absorbers at a dose sufficient to conform precisely to the target volume [21]. Fig. 4 schematically demonstrates the concepts of passive scattering beam and active scanning beam delivery systems used in particle therapy.
3. Issues in beam delivery for particle therapy
In particle therapy, an important issue, based on the respective physical properties, is the range uncertainty in the beam path length. Characterized by steep dose gradients, anatomical changes (including organ movements) might cause an important issue for the robustness of the clinical target coverage. To avoid these uncertainties, charged particle therapy should also include the verification of plan robustness with respect to anatomical changes. Some of the robust optimization methods are distributional robustness, probabilistic robustness, worst case robustness, and voxel-wise worst-case robustness [22].
Another issue for active beam delivery systems is the interplay effect. The interplay effect is a dynamic characteristic of the particle beam that combines body motions (such as breathing) and spatiotemporal difference during beam delivery, resulting in a disagreement between the planned dose and the delivered dose [23]. During the early days of proton therapy for passive beam delivery or fixed target organs, this was not much of an issue. However, in active beam delivery or the scanning-type of beam in advanced systems, this should be considered for moving the target organs such as the lung and liver. As the worst-case scenario, missing of the target organs due to the interplay effect is a concerning issue for radiation oncologists, despite setting up everything perfectly. Nevertheless, researchers reported that the effect could be reduced through multiple fractionation schedules [24]. A study on hepatocellular carcinoma confirmed that proton therapy using pencil-beam scanning with approximately 10 fractions showed no significant effect in terms of local control compared with proton therapy using passive scattering [25]. We can make more robust plans to consider uncertainties such as organ motions by increasing the target margin, or by using planned algorithms to optimize robustness. Also, motion control via abdominal compression or breath-holding might be effective in some patients. Another strategy is real-time tracking of beam delivery. Some tracking methods to tackle the effects of such interplay have been suggested. The most advanced method is “beam gating,” where the irradiation is gated by a pertinent signal generated from the patient. Another system under investigation is the “fast rescanning delivery,” where beam delivery to a homogeneous target irradiation is restored by multiple scans, leading to averaging of the dose inhomogeneity introduced by interplay effects. The last technique is “target tracking,” which aims at real-time tracking of the target motion with the scanning beam. Tracking is the most demanding option because it requires real-time adjustment of not only the lateral beam position but also the beam energy.
Biology in Particle Therapy
1. Linear energy transfer and relative biological effectiveness of particle beams
To explain the radiobiological effect of particle therapy, it is necessary to first define two concepts, namely, linear energy transfer (LET) and relative biological effectiveness (RBE) [21]. When radiation is absorbed by the biological material, ionizations and excitations occur. These are not randomly distributed but tend to be localized along the tracks of individual charged particles in a pattern depending on the type of radiation involved. The LET is the energy transferred per unit length of the track. Usually, high-LET beams are defined as accelerated atomic nuclei with an atomic number greater than 2 due to the different biological effects. Carbon ion (atomic number of 6) beams are defined as high-LET beams. Proton beams are considered intermediary in this regard. Their LET is larger than that of photon beams; however, they still belong to the “radiobiological” group of low LET. Low-LET radiation demonstrates a uniform, sparse spatial distribution of ionization in cells, while high-LET particles bring about dense ionization by depositing energy in the medium, thereby demonstrating higher biological effects. Notably, carbon ions show lower LET in normal tissue and higher LET in the target tissue, yielding a greater therapeutic benefit than other ions, such as oxygen, and protons; oxygen shows relatively high LET in normal tissues, while proton beams show low LET in both tumor and normal tissue areas (Fig. 5).
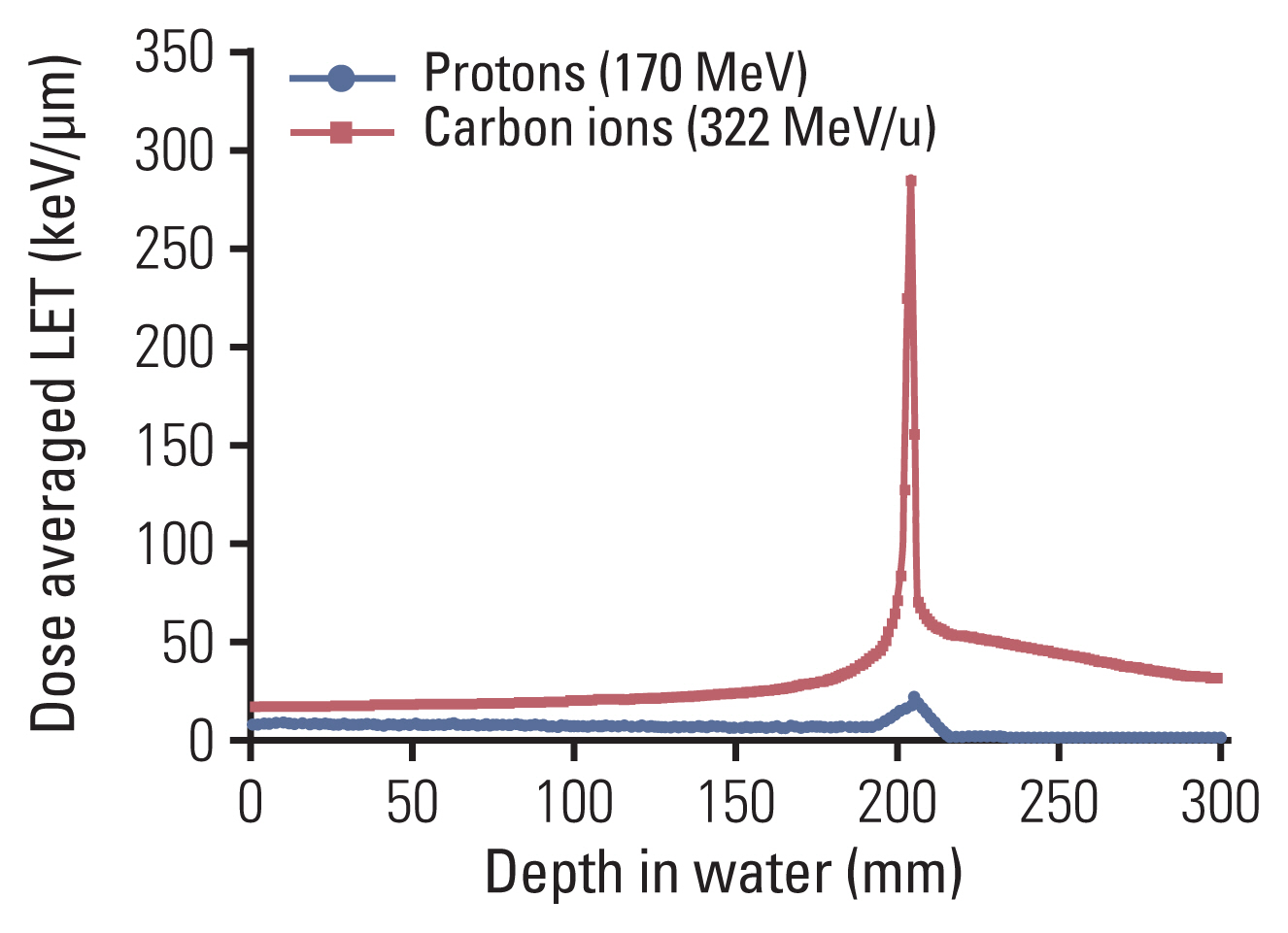
Comparison of dose-averaged linear energy transfer (LETd) between protons and carbon ions in water. The LETd for each particle beam was calculated with the Geant 4 (ver. 10.06) simulation toolkit.
The amount or quantity of radiation is expressed in terms of the absorbed dose, a physical quantity measured in gray (Gy). However, even when physical doses are equal, different types of radiation do not produce equivalent biological effects. For example, 1 Gy of carbon ion beams produces a greater biological effect than 1 Gy of X-rays. When comparing different types of radiations, it is common to use X-rays as the standard. The concept of RBE was introduced to compare the biological effect of different radiation types with that of X-rays. The RBE of a test radiation r is defined as the D250/Dr ratio, where D250 and Dr are the doses of 250-kV X-rays and of test radiation, respectively, required for equal biological effect [26]. For example, suppose we are measuring the RBE of specific particles compared with that of 250-kV X-rays based on the lethality of some specific cells. Groups of cells are irradiated by a range of X-ray doses; parallel groups are irradiated by a range of specific particle beam doses. At the end of the observation, the dose that results in the death of half of the cells in a group turns out to be 9 Gy for X-rays and 3 Gy for the particles. The RBE of the particles is then equal to 3, i.e., the ratio of 9 Gy to 3 Gy.
LET and RBE are closely associated with each other. High-LET radiations usually lead to stronger biological effects than low-LET radiations. RBE is a function of LET. RBE initially increases approximately linearly as LET increases. At higher LET, the increase in RBE slows, eventually reaching a turning point after which RBE decreases, because of energy wasting (Fig. 6). In proton therapy, a generic RBE of 1.1 has been widely used [27], irrespective of dose-modifying factors such as fractionation, tissue type, or radiation quality. An RBE value of 1.1 is a constant scaling factor used along the pathway of the proton, including the normal tissues and tumors; therefore, it does not provide benefits in terms of an increased therapeutic window. However, the actual RBE of the proton beam is known to vary with LET, particularly at the distal part of the range of the monoenergetic proton beam penetration, where LET increases. Thus, it should be avoided to locate critical normal structures at that distal end of the proton beams. In addition, experimental RBE of proton beams is calculated differently depending on the type of cell lines [28]. It was thought that the variance of RBE occurred according to the characteristics of the radiation repair process, and DNA damage response signaling showed different activation durations related to RBE. Thus, the invariant RBE value used currently has been criticized, and optimal ways to replace the standard RBE value are being investigated [29,30].
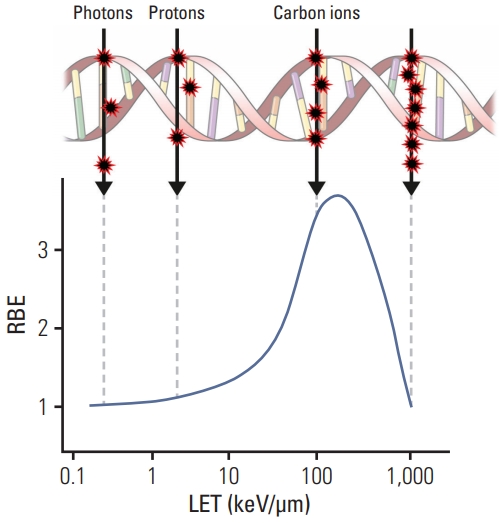
Diagram illustrating why high-linear energy transfer (LET) radiation has the greatest relative biological effectiveness (RBE) for cell death, mutagenesis, or oncogenic transformation. High-LET radiation is most likely to produce a double-strand break from one track for an administered absorbed dose. Beyond the point at which the RBE reaches a peak, energy is wasted because the ionizing events are closer than the diameter of the DNA double helix.
In contrast, the RBE of carbon ions is not a constant value but a function of position within the treatment beam. RBE tends to increase as the particle penetrates deeper into the target lesion. The depth-dose profile for each SOBP field was designed to yield a constant biological effect within the SOBP area by compensating for the increase in LET along its path. Deeper regions in the SOBP, where the LET is high, receive a lower physical dose; in contrast, shallower regions, where the LET is low, receive a higher physical dose (Fig. 7). Furthermore, at the entry site (normal tissue), the RBE value is lower than that in the SOBP, which eventually widens the therapeutic window of carbon ion therapy. In addition, several other radiation dose-modifying factors such as fractionation, tissue type, and radiation quality are taken into account to determine the RBE.
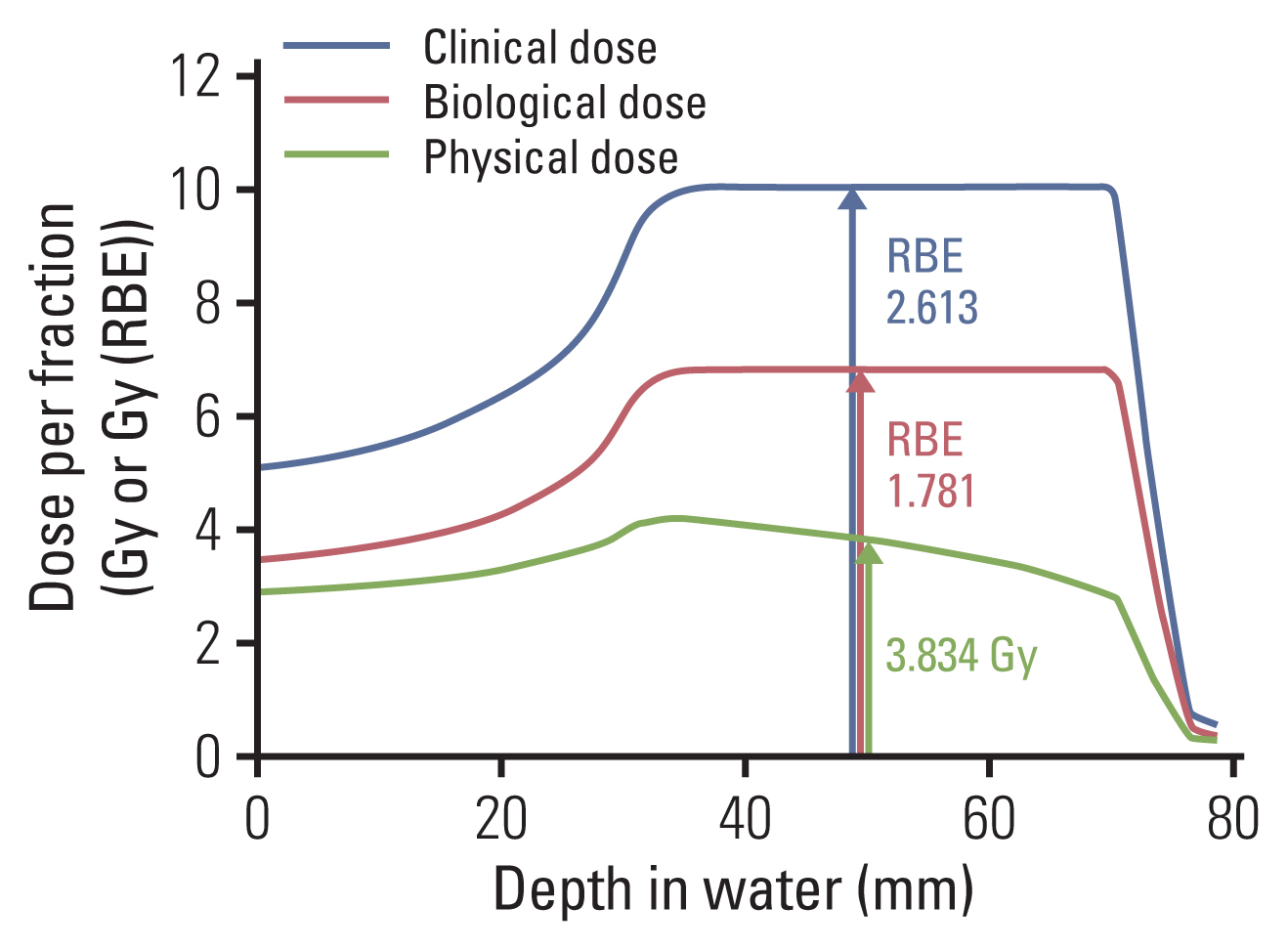
Physical, biological, and clinical depth-dose distributions for carbon beam spread-out Bragg peak (SOBP). The biological model was obtained using an in vitro model of a human salivary gland tumor cell line. The physical dose×relative biological effectiveness (RBE) is supposed to be constant within the SOBP. Note that the physical dose line is curved within the SOBP area. Accordingly, the RBE within the SOBP area is not a constant value but rather a function of depth.
The biological RBE was determined by a specific biological system and a biological endpoint and then scaled to the specific irradiation conditions of patients to obtain a “clinical RBE” (Fig. 7). The clinical RBE describes the ratio of prescribed absorbed doses of a photon to a high-LET irradiation, which are believed to result in clinically equivalent results. The RBE thus obtained was validated using the tumor control probability from clinical data. Because of the complexity of RBE of carbon ion therapy, a biomathematical model is needed to consider the inconstant biological effect appropriate to calculate the RBE in treatment planning. Radiation oncologists have tried to improve the models to reach a “universal” definition of RBE-weighted dose, although it is not yet feasible to fully simulate the underlying biological processes. The use of in vitro data for RBE models is also a major weakness because the biological effectiveness is affected by general patient condition and the tumor microenvironment.
2. Biological characteristics of high-LET radiations
Solid tumors are often characterized by hypoxia. Acute (perfusion-limited) hypoxia is caused by temporary disturbance in perfusion, resulting in fluctuating microvascular oxygen supply. Chronic hypoxia arises due to the over-proliferation of cancer cells with poor vasculature. The increased distance between cells and the nearest blood vessel limits oxygen diffusion from tumor microvessels into the surrounding tissue. Tumor hypoxia is known to correlate with poor prognosis in cancer patients [31]. Low-LET radiation mostly causes DNA damage due to the presence of free radicals, which is enhanced by oxygen. Hence, tumor hypoxia has been considered to be one of the major mechanisms of radioresistance in cancer cells. The ratio of doses to hypoxic and normoxic tissues (oxygen enhancement ratio [OER]) can be close to 3 with low-LET radiation such as gamma-rays and X-rays, making tumor control by radiation difficult in the presence of hypoxia [32]. One method to overcome this obstacle in radiotherapy with low-LET radiation is multiple fractionations. Multiple fractionations allow for the supply of oxygen to the surviving, previously hypoxic volumes, between fractions, through a process termed reoxygenation.
In contrast, high-LET radiation strikes the DNA molecule directly and disrupts the molecular structure. This extensive damage is less influenced by oxygen levels. The OER is lower in high-LET beams. When high-LET radiation is delivered, the hypoxic tumor sites tend to not be affected by oxygen levels, demonstrating similar radiosensitivity. Therefore, heavy ion beams can have a better effect on hypoxic tumors such that the need for fractionation for reoxygenation is diminished. Fractionated irradiation is a basic concept of radiotherapy leading to improved therapeutic ratio. Various biological effects account for the benefits of fractionated irradiation: (1) repair, (2) repopulation, (3) redistribution, and (4) reoxygenation, known as the “4Rs.” Repair and repopulation promote the recovery of damaged normal tissue, and redistribution and reoxygenation promote tumor control. The 4Rs are an important issue in conventional XRT, although they are of little importance for high-LET radiations. For example, sublethal damage repair, which promotes cell survival, is not as obvious in high-LET beams as in low-LET ones. The effect of high-LET radiation is uniform irrespective of the cell cycle, while the effect of low-LET radiation is affected by the cell cycle. The 4Rs’ effects in particle beams are small enough to neglect these factors. For these reasons, carbon ion therapy requires a lower number of fractions, shorter than XRT.
3. Special topics according to biology in particle therapy
Radiogenomics is the study of the link between germline or somatic genetic variations and the clinical variability observed in response to radiotherapy. The Radiogenomics Consortium, which included 133 institutions from 33 countries as of April 2019 [33], has worked on patient samples by using single-nucleotide polymorphisms (SNPs) to perform genome-wide association studies (GWAS). SNPs are DNA sequence variations that occur when a single nucleotide within a gene locus is altered. The principle of a GWAS is to genotype between 300,000 and 1,000,000 tag SNPs, which represent most of the known common variations within the genome. The associations between SNPs and radiation-related toxicity have been identified and validated by the consortium, although the effects are not yet clinically actionable. For example, in prostate cancer, the TANC1 locus at 2q24.1 was found to be associated with overall urinary and bowel toxicity after radiation [34]; the KDM3B locus at 5q31.2 was associated with increased urinary frequency after radiation [35]; and the DNAH5 locus at 5p15.2 was associated with urinary retention after radiation [35].
The radiogenomic data of the response of mammalian cells to charged particles, predominantly protons and carbon ions, is less mature compared with X-ray exposures. Whether patients who receive particle therapy have different biomarkers of normal tissue toxicity than those who receive conventional radiotherapy is an open question. Although radiation generally activates the genes associated with inflammatory pathways, DNA repair, and cell cycle progression, the specific genes activated by X-rays, proton, and carbon ions can be different [36]. Efforts are underway to establish cohorts of patients with prostate cancer treated at the National Institute of Radiological Sciences with carbon ion therapy or proton therapy for radiogenomic studies [37]. Among the results of experimental studies of gene expression after exposure to carbon ions, the downregulation of genes involved in motility due to carbon ion therapy is of particular interest [38,39], which are, in contrast, generally upregulated by X-ray exposure [40–42]. Carbon ion therapy appears to suppress migration, invasion, and metastasis of cancer cells [43,44] and does not lead to the induction of hypoxia-inducible factor-1 [45] and stem cell factor expression [39], both of which are associated with angiogenesis. Reduced tumor cell migration and invasion and reduced angiogenesis may be some of the major benefits of carbon ion therapy. However, the impact of these molecular signatures requires validation in animal models and in human studies.
In addition to dosimetric analyses, tests for germline and tumor genetic variants can be incorporated into clinical decision-making regarding particle therapy. For example, patients who carry the NF1 gene mutation are radiosensitive and predisposed to a number of cancers. NF1 mutations can be used as indicators in patients receiving proton therapy to reduce the risk of secondary radiation-related malignancies [46]. Furthermore, the identification of radioresistance to low-LET radiation using the GWAS approach can improve the selection of patients eligible for carbon ion therapy; however, this approach needs to be validated in prospective clinical studies.
Another special issue with particle therapy is immunotherapy. Immunotherapy has recently emerged as a promising set of new cancer treatments. Immune checkpoint blockade, which increases antitumor immunity by blocking inhibitory checkpoints, has gained an important place in the treatment of various types of cancer. However, the overall response rates with immune checkpoint blockade monotherapy are modest. For example, the response rates of melanoma after ipilimumab and pembrolizumab or nivolumab therapy range from 11% to 19% [47,48] and 33% to 44% [48–50], respectively. Thus, novel strategies that augment systemic immune responses will be potentially critical in the curative management of the disease. The addition of radiotherapy to immunotherapy for patients with predominantly widespread metastases has gained substantial interest. Radiation-induced cancer cell death results in the release of pro-inflammatory signals such as damage-associated molecular patterns danger signals and inflammatory cytokines, thereby triggering the innate immune system to activate tumor-specific T cells. Radiation also has an effect on the tumor microenvironment, by promoting the infiltration of activated T cells, and can overcome some of the barriers to tumor rejection [51]. The advantages and outcomes of combined particle therapy with immunotherapy are still open for investigation. Preclinical studies support the immunogenic potential of proton therapy and suggest that proton therapy may actually have a wider range of immunogenic applications than photon therapy. For example, in vitro studies have reported that protons mediated a greater increase in the expression of calreticulin on the cell surface than photons, increasing cross-priming to cytotoxic T lymphocyte killing [52].
Photon and carbon ion therapies induce different DNA damage and repair pathways, and this difference is based on the differential biological response to low- and high-LET beams. Depending on LET and dose, ionizing radiation causes a variety of different DNA lesions, including single- and double-strand breaks, DNA-protein cross-links, and DNA base damages [53]. This variation can be important because programmed death-ligand 1 expression in cancer cells is upregulated in response to DNA double-strand breaks [54]. Because carbon ion therapy is performed in few sites worldwide, only limited experimental information is currently available. The typical endpoints of these experiments include the abscopal effect, defined as a reaction of the organism’s cells that had not been directly exposed to irradiation, which causes regression of the non-irradiated tumors and the growth of distal metastases [55]. Matsunaga et al. [56] reported that carbon ion therapy administered to a poorly immunogenic squamous cell carcinoma cell line induces reduction of tumor formation after secondary tumor challenge at the contralateral site in mouse models. It has been shown that carbon ion therapy induces systemic antitumor immunity. Combined carbon ion therapy and dendritic cell injection generated more prominent cytolytic activity than carbon ion therapy alone. Ando et al. [57] reported that a combination treatment of carbon ion therapy and intravenous dendritic cell administration enhances the suppression of lung metastases.
Conclusion
Particle therapy involves better dose distribution based on the Bragg peak, physically. Biologically, it shows higher LET and RBE, which is expected to be powerful from a clinical point of view. These properties enable the administration of higher radiation doses to the tumors while subjecting the normal organs to a reasonably low dose, thus widening the therapeutic ratio. Because of the lack of clinical trials, it is not clearly demonstrated whether carbon ion therapy has superior clinical benefits over other radiotherapy modalities. Although further comparison studies are needed, conducting randomized controlled trials comparing carbon ion therapy, proton therapy, and XRT seems to be difficult, mostly because of the differences in treatment costs and patients’ preferences. Nevertheless, we expect that the superior biological and physical aspects of carbon ion therapy will lead to meaningful clinical benefit in cancer patients. Another uncertainty remains concerning the RBE. Currently, the constant RBE value of 1.1 for proton therapy is being criticized; thus, further investigation is required to determine the optimal RBE and its association with LET and dose. The RBE of carbon ion therapy has to be calculated by biomathematical models in treatment planning, which—in spite of all validation efforts—still involve significant sources of uncertainty. Molecular biological research using highly advanced technologies such as multi-omics or the effects of the combination of immunotherapy has to be incorporated into the particle therapy research field. By resolving unsolved issues regarding the physical and biological properties of particle therapy, we believe that the future of particle therapy is promising.
Notes
Author Contributions
Conceived and designed the analysis: Kim JS, Koom WS, Kim YB.
Collected the data: Byun HK, Han MC, Yang K, Koom WS.
Contributed data or analysis tools: Kim JS, Yoo GS, Koom WS, Kim YB.
Performed the analysis: Byun HK, Han MC, Yang K.
Wrote the paper: Byun HK, Han MC, Yang K, Kim JS, Yoo GS, Koom WS, Kim YB.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.