Patterns of Proton Beam Therapy Use in Clinical Practice between 2007 and 2019 in Korea
Article information
Abstract
Purpose
Proton beam therapy (PBT) is a state-of-the-art technology employed in radiotherapy (RT) for cancer patients. This study characterized how PBT has been used in clinical practice in Korea.
Materials and Methods
Patients who received any type of RT between 2007 and 2019 were identified from the radiation oncology registry of the two PBT facilities operating in Korea (National Cancer Center and Samsung Medical Center). The chi-square test was used to identify patient- and treatment-related characteristics associated with the receipt of PBT.
Results
A total of 54,035 patients had been treated with some form of RT in the two institutions, of whom 5,398 received PBT (10.0%). The number of patients who receive PBT has gradually increased since PBT first started, from 162 patients in 2007 to 1,304 patients in 2019. Among all types of cancer, PBT use in liver cancer has been steadily increasing from 20% in 2008–2009 to 32% in 2018–2019. In contrast, that in prostate cancer has been continuously decreasing from 20% in 2008–2009 to < 10% in 2018–2019. Male sex, very young or old age, stage I–II disease, residency in non-capital areas, a definitive setting, a curative treatment aim, enrollment in a clinical trial, re-irradiation and insurance coverage were significantly associated with the receipt of PBT (all p < 0.05).
Conclusion
Since PBT started in Korea, the number of patients receiving PBT has increased to more than 1,000 per year and treatment indications have expanded. Liver cancer is the most common primary tumor among all PBT cases in Korea.
Introduction
Since radiation was first used for cancer treatment in 1886, great progress has been made. Currently, approximately half of all cancer patients receive some type of radiotherapy (RT), either curative or palliative, and when the tumor is localized, the cure rate is more than 50%–60% [1,2]. Among the technological advances made in the last 20–30 years, the clinical application of particle therapy is probably one of the hottest issues in the field of radiation oncology. Particle therapy may have an advantage over other types of RT due to the unique characteristics of particle beams, such as the presence of a Bragg peak, which allows deposition of high doses of radiation within the cancer tissue with much lower doses remaining in the surrounding normal organs [3]. Among the types of particle therapy that can be applied in the current management of cancer patients, proton beam therapy (PBT) is the most widely used technology. Considering the intrinsic physical properties of PBT, it is expected to improve the survival outcomes of cancer patients and their quality of life after treatment. These technological advances in the field of radiation oncology have been changing clinical practice [4]. Initially, clinical application of particle therapy was usually attempted only in challenging cases in which insufficient tumor control was expected with other types of RT or due to the proximity of critical organs, such as in cases of uveal melanoma or skull-base tumor [3]. In studies conducted in the late 1990s and early 2000s, particle therapy proved its efficacy, reaching a local control rate of 90% for the patient groups [5–8]. Since then, the clinical indications for particle therapy have been gradually expanded to include tumors in the head and neck, liver, central nervous system, abdomen, pelvis, and lung. In a recent study, particle therapy significantly lowered the incidence of toxicity compared to other types of RT even in concurrent chemo-RT for locally advanced disease [9].
Particle therapy facilities including PBT for medical purposes have been installed and are operating in approximately 90 locations worldwide as of February 2020, and 30 institutions are preparing to install this technology [10]. In the early 2000s, the Korean government decided to build the first PBT facility in Korea at the National Cancer Center (NCC) to introduce state-of-the-art treatment for cancer management and improve health and welfare, and the PBT facility at the NCC began treating patients in 2007. The second PBT facility has been operating at Samsung Medical Center (SMC) since the end of 2015, and as of 2020, two or more domestic institutions are planning to construct a particle therapy facility using either proton or carbon ions for patient treatment. Despite the recent increased interest in, and expectations for, particle therapy, and more than 10 years of clinical experience of PBT, the impact of these technological advances on the clinical practice of radiation oncology in Korea has not been well documented. Hence, we conducted this study to elucidate PBT use in Korea and identify the determinants of PBT use in radiation oncology departments.
Materials and Methods
1. Study population
A retrospective analysis was conducted using pre-existing deidentified data extracted from the radiation oncology registry of the two PBT facilities operating in Korea (the NCC and SMC). Statistical data extracted from these registries did not include individual patients’ clinical information except parameters related to the current study. All patients who received other types of RT since the initiation of PBT were also identified in each institution. Because the two institutions started offering PBT at different times, the evaluation periods were as follows: NCC, July 2007 to December 2019; and SMC, January 2016 to December 2019. For the data presenting in every 2 years, that of 2007 was excluded because the PBT facility in NCC started its regular operation in July. All patients treated during those periods were included and divided into patients who received PBT and those who received other types of RT. Both institutions are located in the capital area, where 50% of the Korean population resides. SMC is located in the capital city, Seoul, approximately 33 km from the NCC, which is located in a satellite city, Goyang, Gyeonggi Province, west of Seoul. The stage of disease was classified according to the most recent version of the American Joint Committee on Cancer staging system at the time of diagnosis including the 6th, 7th, and 8th version. Age at diagnosis was grouped into four categories: < 40, 40–64, 65–74, and ≥ 75 years. Ethical approval for this study was waived in consultation with the institutional review boards of the relevant facilities, because this study involved the analysis of pre-existing deidentified data.
2. Statistical analyses
Descriptive statistics were generated to characterize the study population according to radiation modality (PBT vs. other types of RT). To identify the determinants of PBT receipt, chi-square tests were used to assess differences in frequency distributions of categorical variables, and odds ratios (ORs) were calculated by analyzing 2×2 cross-table data. Variables assessed as potential determinants included sex, age, tumor stage, patient region, RT setting, RT aim, enrollment in a clinical trial, re-irradiation status, and the type of primary site. Multivariate logistic regression modeling could not be performed as each patient’s individual information could not be captured. p < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS ver. 27.0 (IBM Corp., Armonk, NY).
Results
The total numbers of patients who received PBT in Korea are shown by year with brief summaries of historical events since 2007 in Fig. 1. The numbers of patients have continuously increased since the first PBT facility started operation at the NCC in 2007, whereas those of other types of RT relatively consistent or slowly increasing. The PBT facility at the NCC is equipped with two gantry rooms and one fixed-beam room, and the proton beam is generated by a 230-MeV cyclotron (IBA, Louvain-la-Neuve, Belgium). The most dramatic increases in patient numbers were observed in 2015 and 2016. In September 2015, the Ministry of Health and Welfare of Korea expanded the National Health Insurance System (NHIS) coverage criteria for PBT from childhood cancer alone to some adulthood cancers (central nervous system, head and neck, thoracic and abdominal malignancies except breast and prostate cancer) (S1 Table), as favorable clinical evidence for PBT accumulated, both domestically and overseas [11–16]. Since then, patients have had improved access to PBT, and the use of PBT by medical staff, whether in general practice or for clinical trials, has been possible. SMC began to operate the second PBT facility at the end of 2015. The PBT facility at SMC consists of two gantry rooms, and the proton beam is generated by a 230-MeV cyclotron (Sumitomo Heavy Industries, Tokyo, Japan). The most frequently prescribed doses using proton were more than 80 Gy of 2-Gy equivalent dose and hypofractionation scheme (daily doses of 2–5 Gy and 5–10 Gy) was frequently used. The pencil beam scanning technique, a second-generation PBT, was used in 40% of all PBT cases. Summary of radiation parameters used in PBT are described in the S2 Table.
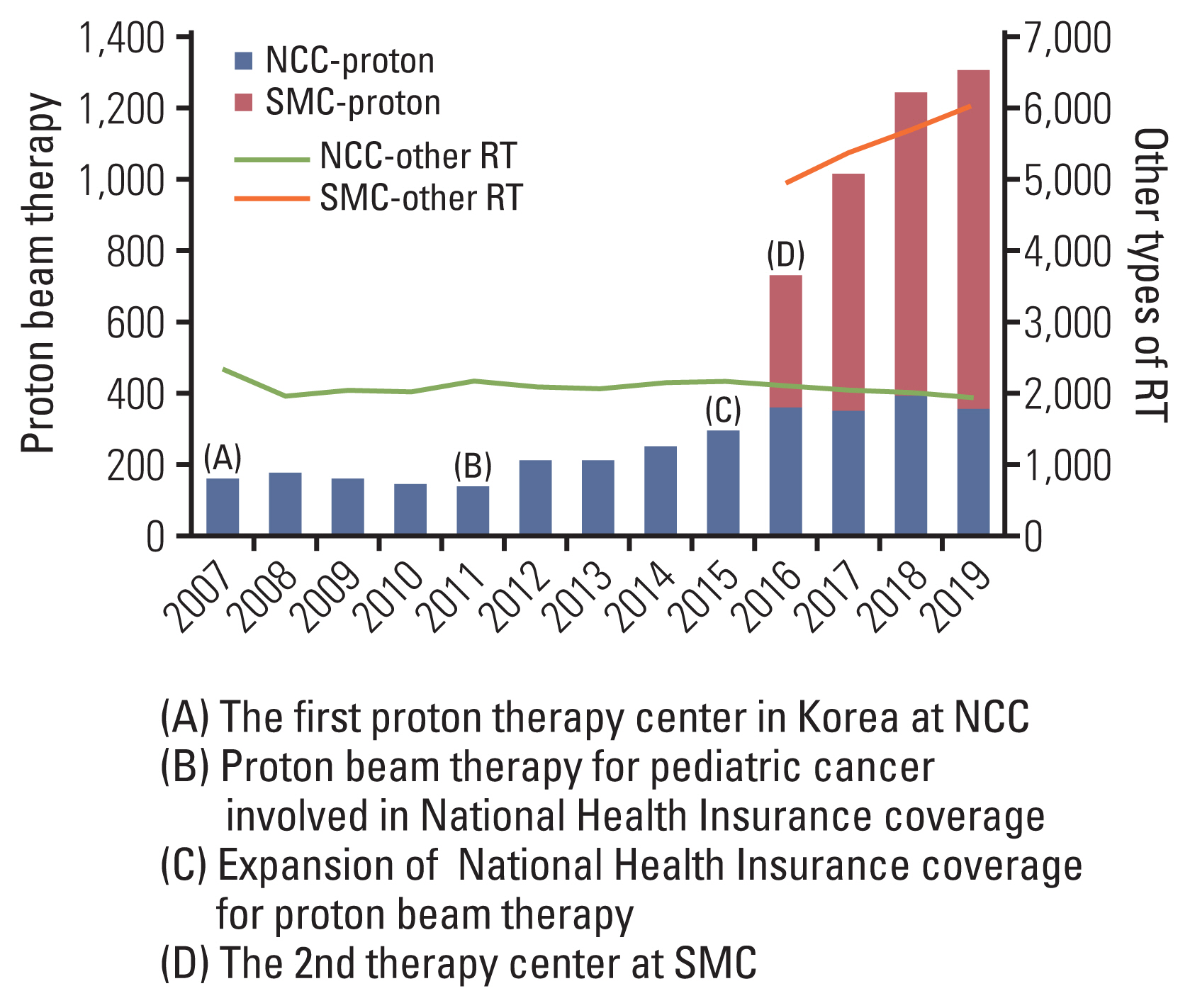
Yearly numbers of patients who received proton beam therapy and timeline of major events related to the use of proton beam therapy in Korea. NCC, National Cancer Center; RT, radiotherapy; SMC, Samsung Medical Center.
In total, 54,035 patients were identified as having been treated with some form of first-course RT in the two institutions during the study period, based on their patient registries. Of these, 5,398 individuals received PBT (10.0% of all patients receiving RT in the two institutions), whereas the remaining 48,637 patients with cancer received other types of RT. The proportions of patients who received PBT among those who received any type of RT for each categorical variable are listed in Table 1. Distributions of sex, age, tumor stage, patient region, RT setting, RT aim, clinical trial enrollment, combination chemotherapy status, re-irradiation status, and primary site type were compared between patients who received PBT and those who received other types of RT. Compared to other types of RT, the PBT group contained significantly more males and very young (< 40 years) or old (> 65 years) patients with a tumor stage of I–II. Regarding treatment-related factors, PBT was administered in a significantly more definitive setting with a curative treatment aim. A significantly larger proportion of patients was treated with PBT in a clinical trial setting, and there were more re-irradiation cases in the PBT group. Liver cancer (27%) was the most common primary malignancy in the PBT group, followed by lung (14%), brain/central nervous system (12%), head and neck (11%), and genitourinary (prostate) malignancies (10%). Among pediatric patients, central nervous system tumor (66.7%) was the most common primary site in the PBT group, followed by soft tissue sarcoma (8.5%). By contrast, female breast cancer was the most common primary malignancy in patients who received other types of RT. In subgroup analysis for pediatric cancer, the PBT group included significantly more patient age < 3-year-old, craniospinal irradiation and brain/central nervous system primary (S3 Table). The ORs of PBT use versus that of other types of RT are listed in Table 2. Male sex, a very young or old age, stage I–II disease, residency in a non-capital area, a definitive setting, a curative treatment aim, enrollment in a clinical trial, re-irradiation, and insurance coverage were significantly associated with the receipt of PBT compared to other types of RT. In terms of primary site, liver cancer (OR, 7.33; 95% confidence interval, 6.29 to 8.54; p < 0.001) had the largest OR of PBT use compared to colon/rectum cancer.

Determinants of proton beam therapy utilization among all cancer patients in radiation oncology department
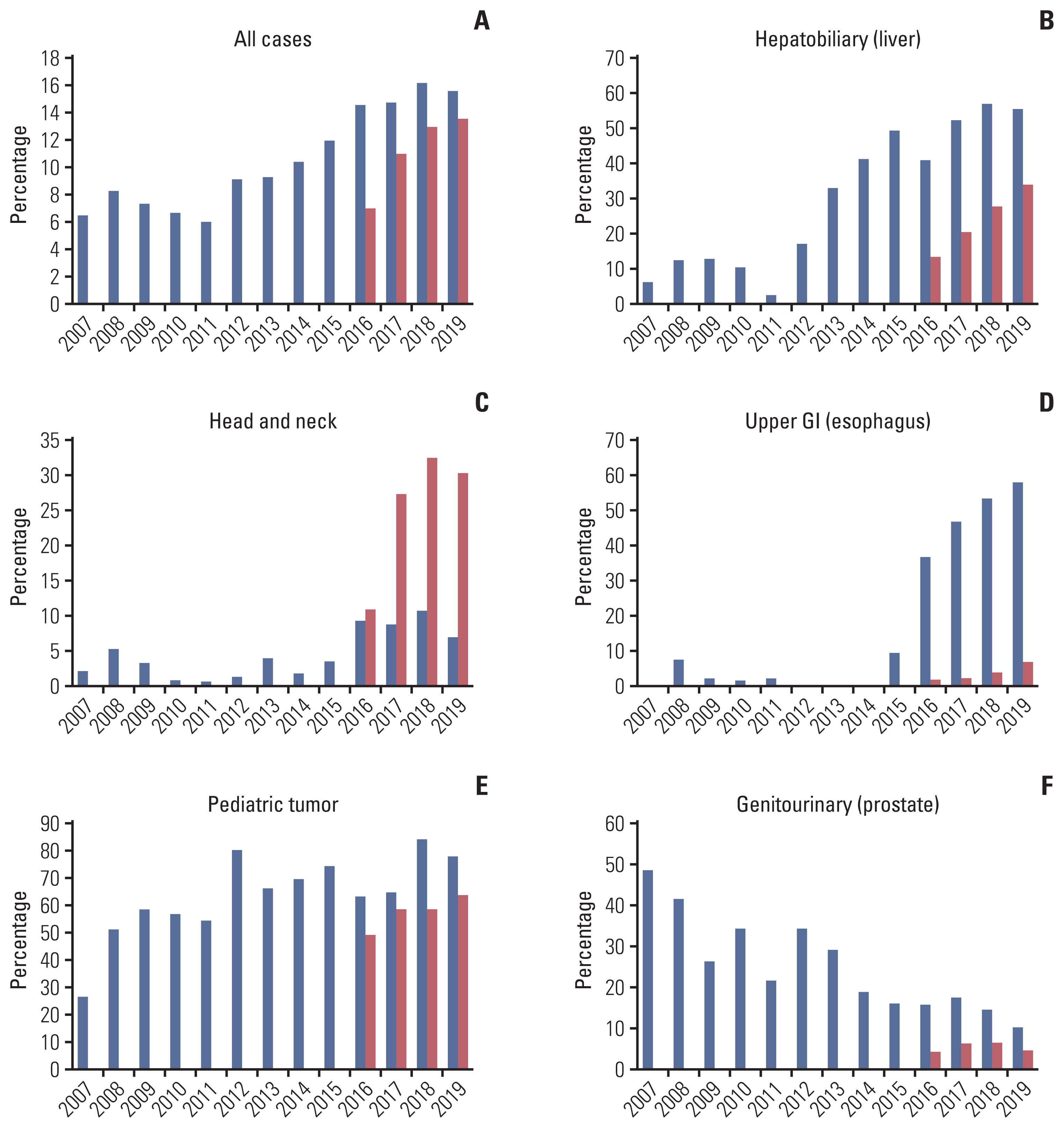
We further assessed the most common primary site at which PBT was frequently used and found that the top-ranked primary site of PBT had changed over time (Table 3). Initially, genitourinary malignancies, as represented by prostate cancer, were the most common primary sites for PBT, followed by hepatobiliary malignancies and lung cancer. However, hepatobiliary malignancies, as represented by liver cancer, had risen to the top by 2014–2015, whereas genitourinary malignancies fell to a rank of third that year and disappeared from the list of the top five most common cancers by 2018–2019. In the case of other types of RT, breast and lung cancer were consistently identified as the most frequent primary cancers during the same period. In Fig. 2, changes in the use of PBT among all RT cases according to primary site by year are depicted. As shown in Fig. 2A, the overall use of PBT has been gradually increasing, from 6% to 16% of all radiation oncology resources. The use of PBT in hepatobiliary, head and neck, and esophageal malignancies has increased notably in the last 3–4 years (Fig. 2B–D). As presented in Fig. 2F, PBT use in prostate cancer has been continuously decreasing from over 40% to below 10%. The use in pediatric cancer seems to be relatively constant (Fig. 2E).

The most common primary site treated by proton beam therapy and other types of RT in two institutions operating proton therapy center in Korea
Discussion
Evaluation of PBT use in a country should be performed in a comprehensive manner that is not limited to clinical aspects, because the use of certain medical resources to manage cancer in society can be influenced by changes in a variety of environmental factors, including governmental policies on health and medical systems, the socioeconomic status of patients, accessibility to medical facilities, and the incidence of certain types of cancers. In this study, we characterized how PBT has been used in clinical practice by assessing changes in PBT use over time and comparing PBT patients’ characteristics with those of patients who received other types of RT in Korea. Since the initiation of PBT in Korea, the numbers of patients receiving PBT have steadily increased to more than 1,000 per year with an overall use rate of approximately 10.0% of all types of RT in the two PBT centers. Not only have clinical experience related to PBT and technological advances increased but there have also been considerable changes in the medical environment, as represented by the expansion of the coverage criteria for PBT by the NHIS. Whether PBT is included under NHIS coverage is critical in the Korean medical system, which is distinctive from those of other countries. Our study is in response to calls from the Korean Cancer Association to improve PBT use across the country and share the experience with other experts.
To confirm the specificity of PBT use in Korea, it is necessary to compare the results of our study with similar studies in other countries. Unfortunately, only a few studies regarding patterns of care using PBT are available to date. Parikh-Patel et al. [17] retrospectively assessed PBT use in California between 2003 and 2016 based on data from the California Cancer Registry. During the study period, California had two operating PBT centers (Loma Linda University and California Protons in San Diego) and one proton ocular center (University of California, San Francisco). Among approximately 600,000 cancer patients who had received some form of RT, 8609 (1.5%) received PBT. Because we analyzed data from the patient registry of two individual institutions and not a nationwide database, a direct comparison of the overall use rate of PBT is not possible. If we consider all RT cases in Korea during the study period, approximately 60,000 per year were estimated to have received some form of RT according to a previous report [18]. During our study period of 2007–2019, a total of 5,398 individuals received PBT, and approximately 420 patients (5,398 patients/13 years) were estimated to have received PBT each year. Overall, the PBT use rate in Korea during the study period could be roughly estimated as 0.7% (420/60,000); however, the numbers of patients receiving PBT have been increasing substantially since 2015–2016, and the rate is expected to rise continuously.
The pattern of PBT use differs depending on the type of primary site. In other countries, prostate cancer may be the most common cancer for which patients receive particle therapy. In California, the most common cancer treated with PBT is prostate cancer (41.3%), followed by breast (14.0%), eye (11.7%), lung (6.1%), and brain (6.0%) cancers [17]. According to a report from the National Institute of Radiological Sciences in Japan describing their 20-year clinical experience of particle therapy using carbon ions [19], prostate cancer (22%) is still the most common primary site type in their institution, followed by cancers of the bone and soft tissue (13%), head and neck (11%), lung (10%), and liver (6%). Prostate cancer has a high incidence rate worldwide, and particle therapy centers using either proton or carbon ions have long promoted this modality for the treatment of prostate cancer [17]. It is worth mentioning Japan’s NHIS because it is similar to that of Korea. Both countries’ NHISs cover particle therapy; however, there are some differences in specific indications [20]. Prostate cancer is within the scope of coverage for particle therapy in Japan’s NHIS [20], but not in that of Korea, which has a relatively wider range of coverage criteria for PBT.
Of note, the most common primary site type in this study was liver cancer (27%), but genitourinary malignancies as represented by prostate cancer (10%) were the fifth-most common primary site type (Table 1). There are several potential reasons for this difference. First, the exclusion of prostate cancer from the scope of NHIS coverage in 2015 probably had a critical impact. This decision appeared to be influenced by an announcement at the time from the American Society for Radiation Oncology that they did not routinely recommend PBT for prostate cancer outside of a prospective clinical trial because clinical benefits of PBT over intensity-modulated RT had been rarely demonstrated [21]. As shown in Fig. 2F, the PBT use rate for prostate cancer has decreased notably since 2014–2015. By contrast, the use rate of PBT for the treatment of liver cancer has been steadily high, since it was included in the scope of NHIS coverage in 2015. Additionally, the relatively high prevalence rate of liver cancer in Korea compared to Western countries would have affected the use of PBT. Several prospective studies have demonstrated the efficacy of PBT in liver cancer [22–25], and recently, the first level I evidence on the use of PBT for the treatment of liver cancer was published by Kim et al. [26]. These results will likely further promote the application of PBT in the future. In the meantime, the application of PBT on head and neck cancer (especially in SMC) or esophageal cancer (especially in NCC) has also increased in recent years. In fact, though it would be difficult to compare clinical policies in using PBT for head and neck cancer in these two institutions in detail, there could be several reasons for high utilization of PBT in head and neck cancer in SMC (Fig. 2C), such as relatively large proportion of those disease in SMC and the starting year of PBT in SMC (after the expansion of the insurance coverage). Regarding esophageal cancer (Fig. 2D), NCC has currently ongoing institutional clinical trial for esophageal cancer, the utilization of PBT for those patients could have been encouraged. Recent technological advances in PBT, including the recent development of the pencil beam scanning technique and a wider field size, also may be one reason for the diversification of PBT indications.
Regarding the determinants for the receipt of PBT, patient and tumor characteristics differed significantly between the PBT group and patients who received other types of RT. Being a patient of male sex, of a very young or old age, with stage I–II disease, and who resided in a non-capital area was associated with a higher OR of receiving PBT versus other types of RT. The most common types of cancer related to PBT, such as liver cancer or prostate cancer, are male-predominant tumors [27], so a significantly higher proportion of cancer patients who received PBT was male (71%) in our study. In general, previous studies have reported that the closer a residence is to a PBT facility, the higher the PBT use rate [17]. However, the distance between patients’ residence and a PBT facility was not a determinant for the choice of PBT in our study, possibly due to the relatively small land area and advanced public transportation systems in Korea [28]. Although we could not assess the socioeconomic status of the patients in this study, several previous studies in other countries have shown that PBT use differs significantly according to demographics and health insurance type. In the United States, being white or male and relatively young, having a higher socioeconomic status and Medicare insurance, and proximity to a facility were significantly associated with PBT use [17]. Ryckman et al. [29] used the National Cancer Database (NCDB) to examine patterns of care associated with PBT use in adult patients with primary brain tumors in the United States. In total, 438 adult brain tumor patients were identified as having received PBT. Several patient-related and socioeconomic factors were significantly associated with the receipt of PBT, including a younger age, being in the highest income quartile, treatment at an academic institution, residence location, diagnosis in more recent years, fewer comorbidities, and non-glioblastoma histological results. Shen et al. [30] also assessed the determinants of PBT use in pediatric cancer in the United States using the NCDB. Similar to the results for adults, socioeconomic factors such as the type of insurance, household income, and educational attainment were significantly associated with increased PBT use. Meanwhile, pediatric cancer patients with metastatic disease were less likely to receive PBT in their study. Those results may not be the case in Korea, possibly because Korea has only one NHIS.
Our study had some limitations that should be considered when interpreting the results. As mentioned above, the study was based on the patient registries of only two PBT facilities in Korea, which were installed at different times. The registries assessed in this study collected information on only the first course of RT. If PBT was administered in a subsequent round of RT, this information would not have been available for this analysis. Also, the difference or the lack of information such as insurance or clinical trials in two separate registries in NCC and SMC prevented further analysis. Regarding patient referral from other hospital for PBT, there was an ambiguous aspect to identifying the proportion of such patients because there can be a variety of reasons other than PBT for patients to change their hospitals. Although it was not able to evaluate in this study, the proportion of patient who came from other hospitals only for PBT is presumed to be not large except pediatric cancer. Despite these limitations, this is the first pattern-of-care study to examine PBT use for all cancer types in Korea. The results can be used as reference material for decision-making regarding the future introduction of particle therapy. There have been notable changes in PBT use over time in Korea, with PBT use differing significantly according to several patient- and treatment-related factors. Constant efforts of medical experts and policy makers in society are required to enhance the evidence-based use of PBT in clinical practice and minimize the number of patients who are marginalized or lack access to this cutting-edge treatment.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
Ethical approval and informed consent for this study were waived in consultation with the institutional review boards of the relevant facilities, because this study involved the analysis of pre-existing deidentified data.
Author Contributions
Conceived and designed the analysis: Lee SU, Yang K, Moon SH.
Collected the data: Lee SU, Yang K.
Contributed data or analysis tools: Lee SU, Yang K, Moon SH, Suh YG, Yoo GS.
Performed the analysis: Lee SU, Yang K, Moon SH, Suh YG, Yoo GS.
Wrote the paper: Lee SU, Moon SH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This study was supported by a National Cancer Center Grant (NCC 2110350-1). The funding source had no role in the study design, data curation, or analysis and interpretation of data.