Identification of a Novel CSNK2A1-PDGFRB Fusion Gene in a Patient with Myeloid Neoplasm with Eosinophilia
Article information
Abstract
Platelet-derived growth factor receptor beta (PDGFRB) rearrangements play an important role in the pathogenesis of eosinophilia-associated myeloid/lymphoid neoplasms. Up to now, more than 70 PDGFRB fusions have been identified. Here, a novel PDGFRB fusion gene CSNK2A1-PDGFRB has been identified in myeloproliferative neoplasm (MPN) with eosinophilia by RNA-sequencing, which has been verified by reverse transcription polymerase chain reaction and Sanger sequencing. The new PDGFRB fusion partner gene CSNK2A1 encoded one of the two catalytic subunit of casein kinase II (CK2). To our knowledge, this is the first report on the involvement of CSNK2A1 in fusion genes, especially fusion with another kinase PDGFRB in MPN. In addition, the CSNK2A1-PDGFRB fusion retained the entire kinase domain of PDGFRB and response to imatinib at low concentration. The patient with CSNK2A1-PDGFRB was sensitive to imatinib treatment and acquired sustained complete remission.
Introduction
On the base of the 2016 World Health Organization, myeloid/lymphoid neoplasms with eosinophilia are commonly related to rearrangements of PDGFRA, PDGFRB, or FGFR1, or PCM1-JAK2 fusion gene [1]. The PDGFRB gene translocation is one of the most chromosomal aberrant in myeloid neoplasms associated with eosinophil [2], high results in the fusion of the 3′ kinase domain of PDGFRB to a 5′ region of the partner gene. So far more than 70 PDGFRB fusions have been reported, mostly reported in single case. Imatinib mesylate function as a tyrosine kinase inhibitor which can potently inhibit ABL kinase, which is equally against PDGFRB kinase, even at a low concentration [3-5]. Most of the patients with PDGFRB fusions show an outstanding long-term response to imatinib treatment at sub-micromolar concentrations [6].
Casein kinase II (CK2) is ubiquitously expressed, constitutively active serine/threonine protein kinase, which was involved in various cellular processes, including cell growth, survival, apoptosis, and circadian rhythm [7]. CK2 upregulated in a lot of malignancies including hematological cancers [8]. The CK2 tetramer consists of two catalytic CK2α and CK2α’ subunits, as well as two regulatory CK2β subunits, with the composed patterns of α2β2, α’2β2 or αα’β2. CK2α was encoded by CSNK2A1 gene (casein kinase II subunit α), which was predominantly studied likely because of its ubiquitous nature. But CK2α’ expressed varied, particularly in brain [9]. All domains of CK2α are highly conserved throughout evolution, but CK2 has low homology with other kinases [10]. In addition, CK2α knock out mice are lethal at E11 with multiple embryonic alterations [11]. Therefore, the importance and uniqueness of CK2α were highlighted. Here, we reported a new fusion gene involving PDGFRB and CSNK2A1 in a pati-ent with myeloproliferative neoplasm (MPN), who is extremely sensitive to imatinib mesy-late treatment. So far, this is the first report on the involvement of CSNK2A1 in fusion genes in cancers.
Case Report
A 37-year-old man was admitted to local hospital with weight loss, night sweat, repeating fever for a week in April 2018. The patient’s initial laboratory examination showed that leukocyte count was 12.48×109/L with 37% eosinophilia in the peripheral blood, hemoglobin concentration was 127 g/L and platelet count was 146×109/L. The initial bone marrow (BM) aspirates and biopsy showed hyper leukocytes and significantly increased eosinophils (8%), and neutrophil alkaline phosphatase score was 18 (Fig. 1A and B). Above all, these inspections were consistent with a diagnosis of MPN. Then the patient was administrated with 20 mg prednisone per day, but it showed no any effect with white blood cell (WBC) 15.1×109/L, hemoglobin 108 g/L, platelet 114×109/L, eosinophils 5.13×109/L (33.91%) in peripheral blood.
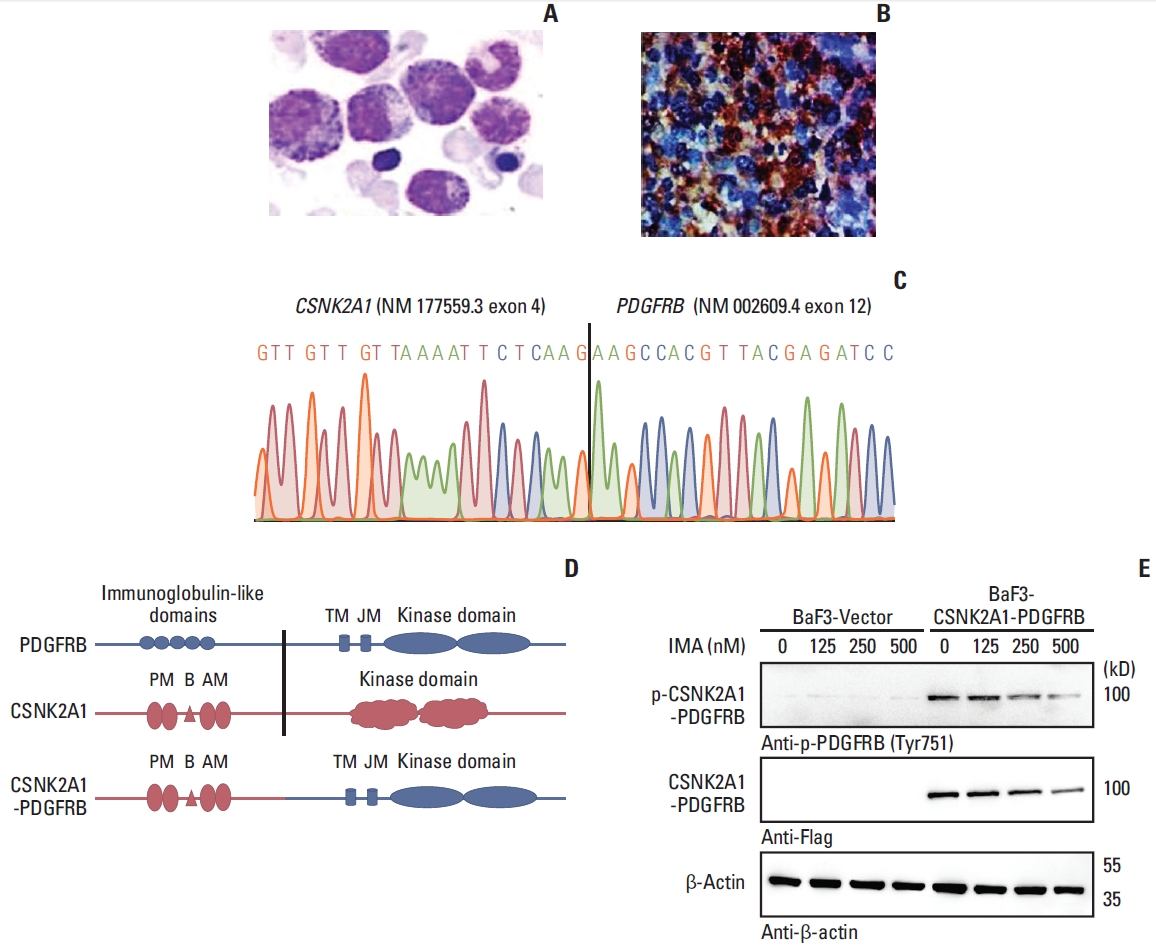
Identification of novel CSNK2A1-PDGFRB fusions. (A) May-Grünwald-Giemsa staining showing several abnormal eosinophilia in the diagnostic bone marrow aspirate. (B) PDGFRB was stained in bone marrow of patient using immunohistochemistry. (C) Sanger sequencing revealed the fusion between exon 4 of the CSNK2A1 gene (NM_177559.3) and exon 12 of the PDGFRB gene (NM_002609.4). (D) Fusion model of CSNK2A1-PDGFRB are shown. (E) Immunoblot analysis show CSNK2A1-PDGFRB is constitutively activated and is inhibited by imatinib in a concentration-dependent manner. AM, ATP binding domain; B, CK2B subunit binding domain; IMA, imatinib; JM, juxtamembrane domain; PM, polypeptide binding domain; TK, tyrosine kinase domain; TM, transmembrane domain.
The suite of fluorescence in situ hybridization (FISH) assay on the BM aspirate was used to detect BCR-ABL, PDGFRA, PDGFRB, and FGFR1 rearrangement. MPN FISH assay showed PDGFRB arrangement positive. The karyotype analysis of BM cells showed 46,XY[20].
Patient’s RNA was extracted from BM cell by Trizol methods according to the manufacturer’s protocol (Invitrogen, Waltham, MA) for RNA-sequencing (RNA-seq) in July 2018. RNA quality and concentration were estimated by Nano Drop ND-2000 (Thermo Fisher Scientific, Waltham, MA). Paired-end reads were generated from the complementary DNA (cDNA) libraries using an Illumina Next Seq 550 instrument (Illumina, San Diego, CA). Then we used star-fusion software to analyze the RNA-seq raw data [12] (Supplementary Material). Standard settings were applied for all three tools and reads were aligned to the Genome Reference Consortium Human Build 37 (GRCh37). RNA-seq revealed that PDGFRB fused with CSNK2A1 gene. To confirm the fusion, reverse transcription–polymerase chain reaction (RT-PCR) was performed with CSNK2A1-PDGFRB forward primer: 5′-GTGCCAAGCAGGGCCAGAGT-3′, reverse primer 5′-AGGGTGCGTCCCAGCACAAG-3′. The reciprocal PDGFRB-CSNK2A1 fusion forward primer: 5′-TCAGAGCTGACACTGGTTCG-3′, reverse primer: 5′-GATGTTGGGACCTCCTCTCAA-3′. The PCR products was purified with PCR purification kit (Tiangen, Beijing, China) and sequenced by GENEWIZ Biotechnology Co., Ltd. (Suzhou, Jiangsu, China). The sequence was analyzed using the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast).
Given the existence of PDGFRB fusion, he received imatinib therapy with 200 mg every day orally in September 2018. Two months later, the laboratory examination showed that WBC was 6.14×109/L with 2.8% eosinophilia in the peripheral blood (S1 Table). Finally, real-time quantitative reverse transcription PCR was performed to quantify the fusion gene and showed negative in 8 months after imatinib treatment. To date, the patient acquired sustained molecular complete remission for 2 years until the last follow-up.
Discussion
RNA targeted capture sequencing showed a fusion between CSNK2A1 exon 4 (NM_177559.3) and PDGFRB exon 12 (NM_002609.4), forming a novel fusion gene CSNK2A1- PDGFRB. RT-PCR and Sanger sequencing has confirmed CSNK2A1-PDGFRB fusion transcripts (Fig. 1C). The reciprocal fusion transcript PDGFRB-CSNK2A1 was negative by detection of RT-PCR. To our knowledge, this is the first case on CSNK2A1 gene rearrangement in neoplasms, especially fusion with another kinase PDGFRB in hematological cancers. The fusion protein retained the transmembrane domain and the entire kinase domain of PDGFRB (Fig. 1D).
Whole cDNA in CSNK2A1-PDGFRB open reading frame was cloned and transduced to BaF3 cells. As a result, CSNK2A1-PDGFRB fusion protein is constitutively activated (Fig. 1E, panel 5). In addition, incubation of BaF3 cells transduced with CSNK2A1-PDGFRB with imatinib for 4 hours caused a concentration-dependent decrease of CSNK2A1-PDGFRB protein (Fig. 1E). The result suggested that imatinib might induce the degradation of PDGFRB fusion protein besides inhibition of its activation.
It is well known that dimerization results in activation of PDGFRB and its down signaling play a vital role in mitogenesis, cytoskeletal rearrangements, and chemotaxis [13]. Most partners have coiled-coil domains, which are required for dimerization or oligomerization of PDGFRB fusions. However, there is no coiled-coil motif in CK2α. In addition, lacking transmembrane domain or disrupting the WW-like domain in juxta membrane region of PDGFRB may also play a role in PDGFRB kinase activation and transformation properties. However, the fusion protein retained the transmembrane domain of PDGFRB. So that, there may be another unknown way to active kinase region of PDGFRB. Notably, CK2α harbored several regions referred to polypeptide binding domain and CK2β binding domain, which were retained in the CSNK2A1-PDGFRB fusion, and may be associated with dimerization or oligomerization of the CSNK2A1-PDGFRB fusion. Indeed, the kinase domain of CSNK2A1-PDGFRB was constitutively activated as shown in Fig. 1E and the patient with CSNK2A1-PDGFRB was sensitive to imatinib treatment.
Totally, we have identified a novel PDGFRB fusion gene with CK2α in an MPN by RNA-seq, which was extremely sensitive to imatinib. To our knowledge, it is the first report to find a CK2α rearrangement in neoplasm, especially fusion with another kinase PDGFRB in MPN.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 221 of 2019 LSP (application)) and was conducted following the Declaration of Helsinki.
Author Contributions
Conceived and designed the analysis: Chen S, Zeng Z, Ruan C.
Collected the data: Xu X.
Contributed data or analysis tools: Xu X, Lu Q, Wang Z, Cai P, Wang M, Ma L.
Performed the analysis: Xu X, Lu Q, Wang Z, Cai P, Zhang L.
Wrote the paper: Xu X, Zeng Z.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This study was supported by grant from the National Key R&D Program of China (2019YFA0111000), the National Natural Science Foundation of China (81700140, 81873449, 81970142, 81900130, 81970136, 82000132), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (18KJA320005), the Natural Science Foundation of Jiangsu Province (BK20190180), China Postdoctoral Science Foundation (2018M632372), the priority academic program development of Jiangsu Higher Education Institution, Translational Research Grant of NCRCH (2020WSB11, 2020WSB13).