Targeting Hypoxia Using Evofosfamide and Companion Hypoxia Imaging of FMISO-PET in Advanced Biliary Tract Cancer
Article information
Abstract
Purpose
Hypoxia is widely known as one of the mechanisms of chemoresistance and as an environmental condition which triggers invasion and metastasis of cancer. Evofosfamide is a hypoxia-activated prodrug of the cytotoxin bromo-isophosphoramide mustard conjugated with 2-nitroimidazole. Biliary tract cancer (BTC) is known to contain large hypoxic area. This study evaluated the efficacy and safety of evofosfamide as a second-line treatment of advanced BTC.
Materials and Methods
Patients received evofosfamide at a dose of 340 mg/m2 on days 1, 8, and 15 of every 28-day cycle. Primary end-point was progression-free survival (PFS) rate at 4-months (4m-PFSR). Secondary end-points included overall survival (OS), PFS, disease control rate (DCR), metabolic response by 18F-fluorodeoxyglucose positron emission tomography (PET), hypoxic parameters evaluated by 18F-fluoromisonidazole (FMISO) PET and toxicity.
Results
Twenty patients were treated with evofosfamide, with 16 response-evaluable patients. There was no objective response; stable disease was observed in nine patients, with a DCR of 56.25%. 4m-PFSR was 40.6%. Median PFS was 3.60 months (95% confidence interval [CI], 1.68 to 5.52). Median OS was 6.37 months (95% CI, 3.94 to 8.79). Reduction of tumor metabolic activity was observed in eight of 15 patients (53.3%). High baseline hypoxic parameters were associated with poor PFS. Change of hypoxic parameters between pretreatment and post-treatment reflected hypoxic-activated drug response. There was no treatment-related death.
Conclusion
Evofosfamide as second-line treatment of advanced BTC showed acceptable safety and comparable efficacy to other agents. Changes in volumetric parameters measured with FMISO PET, showing the degree of tumor hypoxia, reflected the response to evofosfamide based on the mode of action.
Introduction
Biliary tract cancer (BTC) is a relatively rare tumor with a poor prognosis. Its incidence is higher in Asia than in Western countries [1,2]. Most patients with BTC are diagnosed in the advanced stage due to the absence of adequate screening methods in the early stage and the high recurrence rate after curative surgery [3–5]. Currently, gemcitabine/cisplatin combination chemotherapy (GemCis) is the standard of care as first-line treatment for advanced BTC, but its overall survival (OS) is around 11 months [6]. Despite 5-fluorouracil (5-FU) based chemotherapies being commonly used as salvage treatment [4], there is no established chemotherapy after progression to GemCis. Therefore, there is a huge unmet medical need in advanced BTC.
Hypoxia is widely known as one of the mechanisms of chemoresistance in solid tumors and as an environmental condition which triggers invasion and metastasis of cancer [7]. Some preclinical studies suggested that hypoxia is a major cause of the aggressive phenotype of cholangiocarcinoma [8] and may be one of the resistant mechanisms to gemcitabine [9].
Evofosfamide (TH-302), a hypoxia-activated prodrug and a nitroimidazole-linked prodrug of a brominated derivative of an isophosphoramide mustard, acts as a DNA alkylator in hypoxic environment [10–12]. In normoxia, Evofosfamide remains intact as a prodrug and toxicity is minimized. Tumors often consist of large areas of highly hypoxic regions that are resistant to chemotherapy and radiotherapy [7]. Thus, evofosfamide has been designed to target these highly hypoxic tumor regions; marking it an attractive candidate for clinical development [10]. Hence, it would be worth testing evofosfamide, which targets tumor hypoxia, as a second-line treatment in advanced BTC.
Materials and Methods
1. Study design
This study was a prospective, single-center, open-label, single-arm, phase II trial aiming to evaluate the efficacy and safety of Evofosfamide monotherapy as a second-line treatment in advanced BTC patients. Participants diagnosed with initially unresectable or metastatic/recurred BTC were enrolled. Details of inclusion and exclusion criteria were described in the Supplementary Method.
2. Treatment and dose modification
Evofosfamide was administered at a dose of 340 mg/m2 via intravenous infusion over 30 minutes on days 1, 8, and 15 of every 28-day cycle. In the beginning of each cycle, absolute neutrophil count, adequate hemoglobin, and platelet level were assessed in each patient. Dose reduction of evofosfamide up to 255 mg/m2 was allowed based on the hematologic toxicity. Treatment was repeated every 4 weeks until disease progression, unacceptable toxicity, or patient’s consent withdrawal.
3. Positron emission tomography/computed tomography protocol
18F-Fluoromisonidazole (FMISO) positron emission tomography (PET)/computed tomography (CT) was the non-invasive modality for imaging oxygen-deprived hypoxic cells. This involved using an intracellularly-accumulated isotropic fluoro-nitroimidazole compound after the reductive reaction in the hypoxic environment [13,14]. The patients underwent FMISO PET/CT using dedicated PET/CT scanners (Biograph 40 True-point, Siemens, Knoxville, TN). FMISO of 555 MBq was administered intravenously, and image acquisition was started at 180 minutes after the injection. PET scan was acquired after the CT scan in the static mode. PET images were corrected for attenuation and reconstructed onto a matrix of 200×200 using the three-dimensional ordered-subsets expectation maximization algorithm (2 iterations, 21 subsets, 5-mm Gaussian filter).
18F-Fludeoxyglucose (FDG) PET/CT was performed using the same PET/CT scanners. After fasting for at least 6 hours, FDG of 5.18 MBq/kg was administered intravenously, and image acquisition was started at 60 minutes after the injection. Serum glucose levels were less than 150 mg/dL at the time of FDG administration in all patients. The reconstruction method was the same as FMISO PET/CT.
4. Assessment
After written informed consent was obtained, participants were evaluated for trial eligibility and baseline assessments were conducted. Tumor assessments were conducted using the Response Evaluation Criteria in Solid Tumor (RECIST) criteria ver. 1.1 every 8 weeks. Metabolic response was evaluated with the European Organization for Research and Treatment of Cancer (EORTC) response criteria using FDG PET at screening and after 8-week treatment. FMISO PET was performed at screening and after 8-week treatment to measure the degree of hypoxia in tumors. Subsequently, standardized uptake value (SUV), hypoxic volume (HV), hypoxic lesion uptake (HLU), and tumor to background ratio (T/B) which are representative parameters for hypoxia in tissue, were measured. HV means tumor volume with SUV in FMISO PET equal to or greater than a hypoxic region threshold. Background SUV was used as SUVblood to determine the cutoff. Considering both the value of threshold in the previous studies [15] and the result of background SUV from our data, the cutoff value we selected was 1.6, which was able to distinguish FMISO absorption from surrounding tissues well. HLU was calculated as product of HV and average SUV. The HLU, implying actual hypoxia in tumor, has similar concept with the total lesion glycolysis (TLG) of FDG PET. Blood pool uptake was calculated as product of the investigator-defined volume (0.5 cm3) and SUVblood. T/B was the ratio of the HLU of the tumor to the blood pool uptake, unlike the previous studies [15,16], that represented hypoxic tumor volume and amount of hypoxic tracer uptake. Toxicity was evaluated at each cycle using the Common Terminology Criteria for Adverse Events ver. 4.03.
5. Statistical analysis
The primary end-point of this trial was progression-free survival (PFS) rate at 4-month (4m-PFSR). Secondary end points were objective response rate (ORR), disease control rate (DCR), PFS, OS, metabolic response measured by FDG PET, hypoxic parameters evaluated by FMISO PET and toxicity. PFS was calculated from the date of study enrollment to the first date of documented progressive disease (PD). OS was defined as the period from the date of study enrollment to the date of death. PFS and OS were estimated using the Kaplan-Meier method. The Wilcoxon signed rank test and the Mann-Whitney U test were used to compare changes in hypoxic parameters. Comparison of values evaluated by FMISO PET and FDG PET was assessed using the Spearman’s rank correlation coefficient. Selected cutoff value of hypoxic parameters was calculated using maximally selected rank statistics.
Results
1. Patient characteristics
Between April 30, 2015, and October 13, 2016, 24 patients were enrolled. Two patients were excluded due to screening failure, and two patients withdrew consent before dosing. Finally, a total of 20 patients were treated with evofosfamide. Baseline characteristics of the patients are summarized in S1 Table. The median age was 58.73 years, and 13 patients (65%) were male. All patients received prior GemCis [6], and 16 patients had Eastern Cooperative Oncology Group performance status of 0.
2. Efficacy
In 16 patients, response was evaluable. One out of four patients who were unable to evaluate the response died of disseminated intravascular coagulation (DIC) after 1 dose of evofosfamide treatment, and the other three withdrew consent before completing the second cycle treatment. There was no objective response, nine patients had stable disease (SD, 56.25%), and seven had PD (43.75%). The ORR was 0% and the DCR was 56.25%. The protocol-specific primary end-point of 4m-PFSR was 40.6%, and PFS was 3.67 months (95% confidence interval [CI], 1.31 to 6.03). The OS was 6.13 months (95% CI, 3.43 to 8.84). The 12-month-survival rate was 15%. After 8 weeks, reduction of tumor metabolic activity by FDG PET was observed in eight out of the 15 patients (53.3%). Two patients had partial metabolic response evaluated by the EORTC response criteria. Patients with partial metabolic response were evaluated as SD according to RECIST.
3. Prognostic value of tumor hypoxia
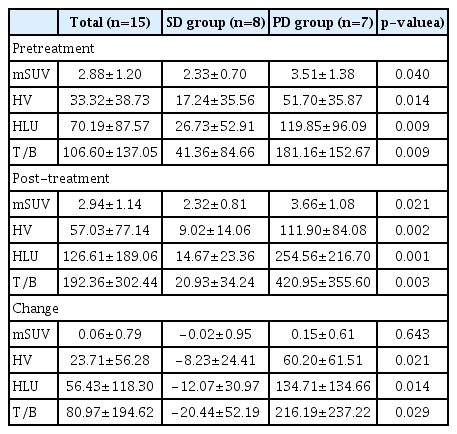
Among the response-evaluable 16 patients, the degree of hypoxia in tumor tissue was evaluated using FMISO PET in 15 patients. One patient was excluded because of the absence of appropriate target lesion on FMISO PET. The degree of hypoxia in the target lesion was related to the prognosis. All of the hypoxic parameters before evofosfamide treatment were higher in the PD group than in the SD group (Table 1). High HV, HLU, and T/B before evofosfamide treatment were associated with worse PFS (Fig. 1). The selected cutoff value of pretreatment HV estimated by maximally selected rank statistics was 4.93 (p=0.013), and pretreatment HLU was 3.50 (p=0.026). Other estimated cutoff values, such as maximum standardized uptake value (mSUV) of FMISO PET and others of FDG PET, were not statistically significant. In the group with high baseline HV, HLU, and T/B, a shorter OS trend was also observed, but not statistically significant (S2 Fig.).
The association between tumor hypoxia and prognosis. The cutoff value of hypoxic parameters measured by 18F-fluoromisonidazole positron emission tomography (FMISO PET) was obtained using maximally selected rank statistics. Progression-free survival (PFS) was statistically significantly longer in patients with low hypoxic parameters as measured by FMISO PET before evofosfamide treatment. (A) Patients with high maximum standardized uptake value (mSUV) measured using FMISO PET before treatment showed a shorter PFS than those with low mSUV values (2.07 months vs. 3.80 months, p=0.328). This was not statistically significant. (B) Patients with high hypoxic volume (HV) measured by FMISO PET before treatment showed a statistically significantly shorter PFS than those with low HV (1.97 months vs. 7.23 months, p=0.001). (C) Hypoxic lesion uptake referred to the value of HV multiplied by the average SUV value, and PFS was statistically significantly shorter than that of the HV group (2.07 months vs. 7.23 months, p=0.008).
4. The association between changes in tumor hypoxia and tumor response
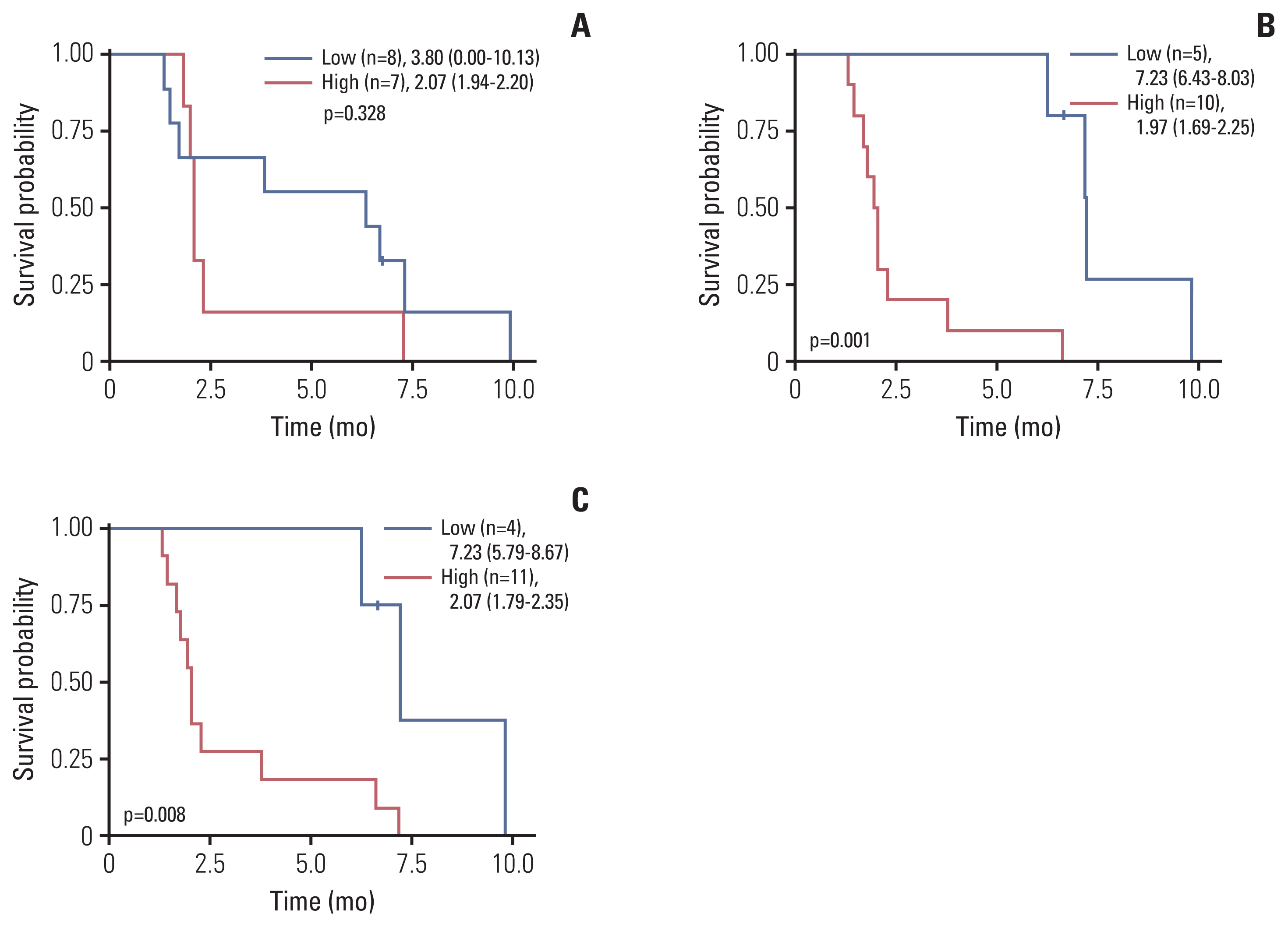
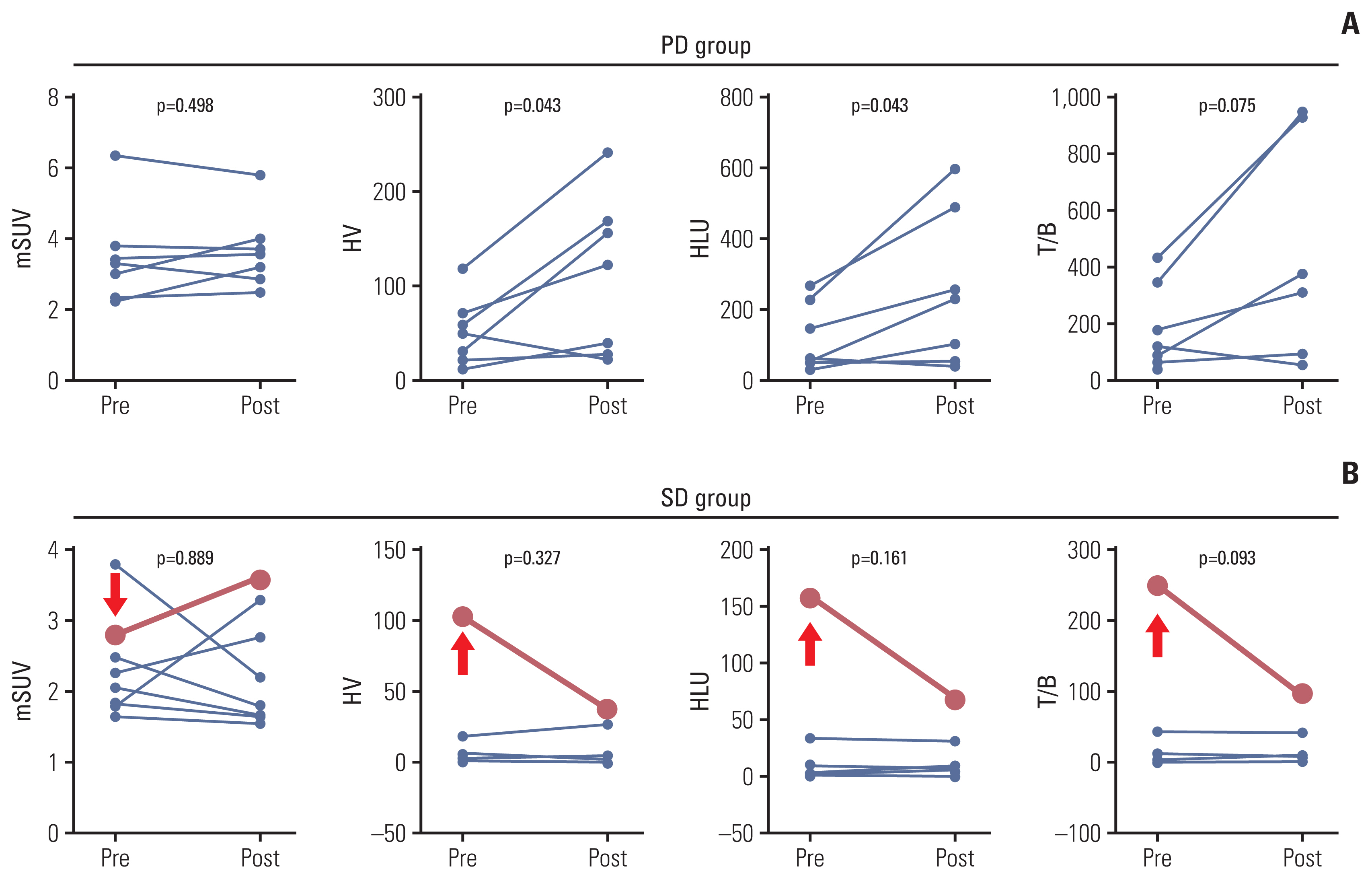
With the treatment of evofosfamide, hypoxic parameters showed dynamic changes (Table 1, Fig. 2). All of the hypoxic parameters were decreased in the SD group after evofosfamide treatment, but not in the PD group. Comparing the tendency of hypoxic parameters changes, the degree of hypoxic change between the two groups was statistically significant (Table 1). Interestingly, in the PD group, the hypoxic indexes tended to increase after treatment, suggesting that tumor hypoxia might have worsened when the tumor progressed (Fig. 2A). Additionally, increases in volumetric parameters such as HV and HLU after evofosfamide treatment showed a trend associated with worse PFS (Fig. 3).
The association between changes in tumor hypoxia and tumor response. (A) Parameters indicating the degree of hypoxia on 18F-fluoromisonidazole positron emission tomography showed a tendency to increase in patients with progressive disease (PD) after evofosfamide treatment. Statistical significance using Wilcoxon signed rank test was shown only in some parameters: hypoxic volume, hypoxic lesion uptake. (B) There was no significant change in the hypoxemia parameter in the stable disease (SD) group. HLU, hypoxic lesion uptake; HV, hypoxic volume; mSUV, maximum standardized uptake value; T/B, tumor to background ratio.
The association between changes in tumor hypoxia and progression-free survival. (A) Patients who showed increased maximum standardized uptake value measured by 18F-fluoromisonidazole positron emission tomography (FMISO PET) after treatment showed shorter progression-free survival than patients who did not. This was not statistically significant (1.80 months vs. 2.30 months, p=0.281). (B) Progression-free survival was shorter as the difference of hypoxic volume (HV) values measured by FMISO PET before and after treatment increased (1.97 months vs. 6.63 months, p=0.063). (C) Similar trend as HV was observed in the result of the comparison of hypoxic lesion uptake (2.07 months vs. 6.27 months, p=0.166).
5. Comparison between FMISO PET and FDG PET
ontrary to the results of FMISO PET, metabolic parameters assessed with FDG PET/CT prior to evofosfamide treatment did not differ between SD and PD groups (S3 Table). However, metabolic tumor volume and TLG as the volumetric parameters evaluated by FDG PET tended to increase in the PD group after evofosfamide treatment, similar to the results of HV and HLU in FMISO PET. Besides, there was a positive association between the volumetric indexes of FMISO PET and FDG PET, and this association was more robust after evofosfamide treatment compared to before (Fig. 4, S4 Table).
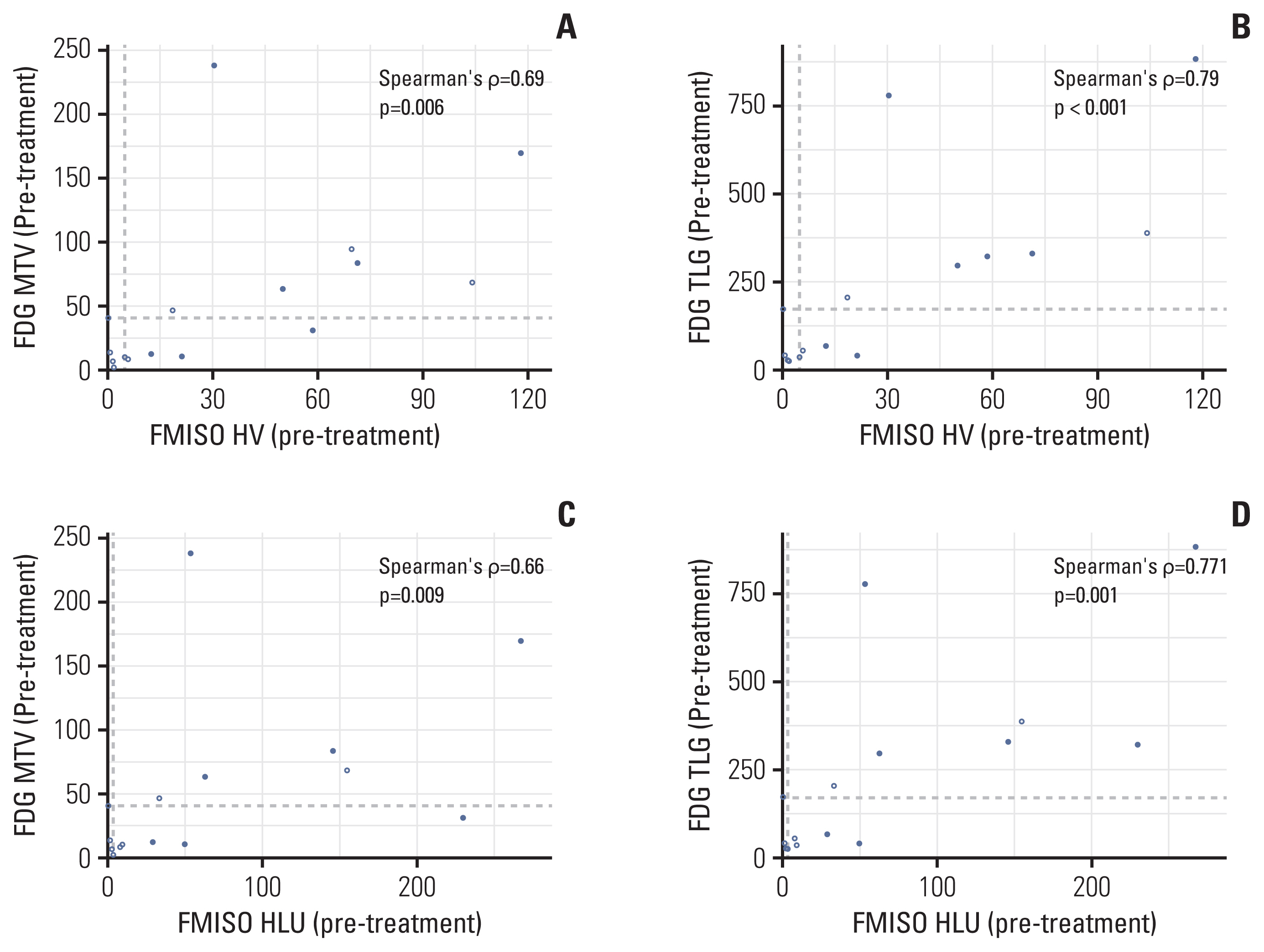
Comparison of values evaluated using 18F-fluoromisonidazole positron emission tomography (FMISO PET) and 18F-fludeoxyglucose (FDG) PET before treatment. Spearman’s rank correlation coefficient was conducted to compare values of functional imaging tools. Values were considered significant for p < 0.05. White and black circles indicate patients with stable disease (n=8) and with progressive disease (n=7), respectively. (A, B) Hypoxic volume (HV) in FMISO PET was significantly associated with metabolic tumor volume (MTV) and total lesion glycolysis (TLG) in FDG PET. (C, D) Hypoxic lesion uptake (HLU) in FMISO PET was significantly associated with MTV and TLG.
We estimated the accuracy about prognosis using evofosfamide in both PET. The accuracy of hypoxic parameters in FMISO PET was 73.3%–80%, but metabolic parameters in FDG PET was 46.7%–73.3%. Thus, hypoxic parameters by FMISO PET may better reflect disease progression than metabolic parameters.
6. Representative case series
These are representative cases that can show the association between the response to evofosfamide and the change of hypoxic parameters assessed by FMISO PET. Patient A (S5A Fig.) initially showed high levels of hypoxic markers that reached the PD group’s mean level. That patient was evaluated as an SD according to the RECIST, and partial metabolic response by FDG PET. Most of the hypoxic parameters decreased after evofosfamide treatment (Fig. 2B, arrow).
Patient B (S5B Fig.) and patient C (S5C Fig.) are both evaluated as a PD after evofosfamide treatment, according to the RECIST. However, in patient B, the hypoxic parameter before treatment was evaluated as the mean level of the SD group. After evofosfamide treatment, hypoxic parameters in this patient were remarkably increased. The response of evofosfamide in this patient was assessed as a PD in all response evaluations using CT, FDG PET, and FMISO PET. On the other hand, patient C was evaluated as a PD according to the RECIST and FMISO PET. However, metabolic mSUV in FDG PET was decreased, which means the hypoxic indexes rather than metabolic parameters reflect the disease progression considering the mode of action of evofosfamide.
7. Toxicity
Patients underwent a total of 34 cycles of treatment. Study treatment was discontinued in four patients (20%) because of adverse events including duodenal ulcer bleeding, soft tissue infection, hyponatremia, and DIC. These patients could not resume evofosfamide due to their poor performance status after the adverse events had subsided. One patient (5%) required dose reduction due to thrombocytopenia. No treatment-related death occurred. Detailed adverse events per patient are described in S6 Table. The most common adverse event was anemia (32.5%), followed by thrombocytopenia (20%), neutropenia (15%), and nausea (7.5%). The most common grade III adverse event was anemia.
Discussion
This study suggests that evofosfamide might be a treatment option in patients with advanced BTC. The 4m-PFSR was 40.6%, and PFS and OS were 3.67 months (95% CI, 1.31 to 6.03) and 6.13 months (95% CI, 3.43 to 8.84), respectively. DCR was 56.25%. Though there is no objective response to evofosfamide in this study, evofosfamide has comparable PFS and OS efficacy with 5-FU–based chemotherapies in second-line treatment and was better than other biologics such as sunitinib [17], and sorafenib in advanced BTC [4,18].
Most adverse events were either grade 1 or 2. Grade 2 or 3 toxicities were easily managed and dose reduction due to toxicity was required in only one patient because of grade 2 thrombocytopenia. Four patients discontinued treatment due to adverse event (duodenal ulcer bleeding, soft tissue infection, hyponatremia, DIC); however, these adverse events were not related with evofosfamide. Thus, evofosfamide treatment showed comparable safety profile with other cytotoxic chemotherapies and biologics as a second-line treatment in advanced BTC [4].
With the development of chemotherapy, the concept of tumor hypoxia emerged as one of the resistance mechanism of anti-cancer therapy [7]. Concerning this, tools such as FMISO PET that objectively measures tumor hypoxia might be intriguing because they define whether the tumor is hypoxic or not. Previous studies [13,19] suggested that prognosis in patients with head and neck cancer or glioblastoma was poor when FMISO PET uptake in tumor before treatment was high. Here, we report that advanced BTC patients with high tumor hypoxia measured by FMISO PET had a tendency of poor prognosis (Fig. 1, S2 Fig.). It is appropriate for evaluating the prognosis with any biomarker or imaging tool to verify whether there is a difference in OS. However, unfortunately, there was no statistically significant difference in OS (S2 Fig.). It was challenging to obtain differences in OS due to the study population’s characteristics because of the limitations of a second-line setting. Moreover, considering the mode of action of evofosfamide, it is difficult to predict the drug response with FMISO uptake before treatment alone. Therefore, we speculated that the degree of FMISO absorption before treatment might reflect the prognosis, and further studies are needed in advanced BTC to precisely verify whether the pretreatment hypoxic parameter reflects the prognosis as with other tumors.
Evofosfamide has an interesting mode of action, that is, this is prodrug itself, and is activated in hypoxic condition. Based on its mode of action, we tried to find out association between clinical activity and dynamics of tumor hypoxic parameters. Intriguingly enough, an increase in hypoxic parameters was observed more predominantly in the PD group than SD group (Table 1). In parallel, increases in volumetric hypoxic parameters after evofosfamide treatment might be associated with worse PFS (Fig. 3). Furthermore, from the results of patient A, we could expect that the hypoxic parameter may decrease if there is a response to evofosfamide. Patient A had a relatively high baseline hypoxic parameter before treatment. However, the hypoxic parameters significantly decreased after two-cycles of evofosfamide treatment (Fig. 2B, arrow), and the patient showed prolonged disease stabilization with PFS of 6.63 months and OS of 17.57 months. Suggesting that evofosfamide targets tumor hypoxia based on the mode of drug action, leading to a reduction of hypoxic lesions, which was beneficial to the patient. Similarly, in other patients with the response of SD, the hypoxic parameters were slightly reduced with evofosfamide during disease control. Therefore, tumor response to hypoxic targeting drugs could be more accurately captured by functional FMISO PET than CT. Early resolution of FMISO uptake during treatment was reported to be associated with excellent loco-regional control in head and neck cancer patients treated via radiotherapy [20], similarly, our result also shows that changes in hypoxic index accurately demonstrate disease status and response prediction.
Between both functional imaging tools, there are relatively good positive correlations in volumetric parameters (Fig. 4). The insufficient association between SUVs has already been reported in the previous studies [21,22]. We reported that volumetric parameters in both modalities reflected disease progression, and hypoxic volumetric parameters are more accurate than metabolic parameters in assessing disease progression. This suggests that quantitative rather than qualitative variables in functional imaging tools may be more useful in assessing tumor response.
On the contrary, the response to Evofosfamide and the metabolic response by FDG PET were inconsistent, as shown in two other representative cases (S2B and S2C Fig.). If FDG PET quantifies the degree of tumor metabolism as an image, FMISO PET quantifies the degree of tumor hypoxia. Tumor metabolism and hypoxia are important for cancer progression and invasion, but as previously confirmed in other study [23], both tests provide complementary information to each other and are not always consistent. Considering the cases of patients B and C, they showed increases in hypoxic parameters in FMISO PET. However, patient B who was evaluated as PD on FDG PET showed shorter OS than patient C who had metabolic response. So, volumetric parameters in functional imaging tool may represent differences in their survival outcomes, hence, further studies about volumetric parameters in functional imaging are required.
FMISO PET is an intuitive imaging tool that is useful to evaluate hypoxia, but it is not yet widely applied. One of challenging things of its usage is that 18F has a half-life of approximately 109 minutes but hypoxic tissue requires an uptake period of 2–4 hours. Although FMISO is the most widely investigated radiotracer for hypoxia evaluation, limitations of FMISO such as the long image acquisition time and the low signal-to-noise ratio which requires a minimum of 2 or optimally 4 hours between tracer injection and scanning has led to the development of new imidazole derivatives. Therefore, the research community worked on the development of other radioactive tracers to overcome the shortcomings of 18F-FMISO, and second/third generations hypoxia tracers began to be developed. Of the greatest interest, 18F-fluoroazomycin-arabinoside (FAZA) was developed to avoid the high lipophilic drawback of FMISO. A comparative study showed that 18F-FAZA was more sensitive to acute hypoxia than 18F-FMISO. However, it is true that the 18F-FAZA image, as in all other hypoxic PET cases, is still difficult to interpret because its contrast with normal tissue is low [24]. The reasons mentioned make us look forward to the development of another hypoxic tracer.
Evofosfamide as a second-line treatment of advanced BTC showed acceptable safety and comparable efficacy to other agents in terms of disease stabilization, PFS and OS. Degree of tumor hypoxia measured using FMISO PET reflected the prognosis and changes in volumetric parameters measured with FMISO PET reflected the response to hypoxia-targeting agents based on the mode of action. This study addresses potential of further clinical development of hypoxia-targeting strategy for new drug development in solid tumors with companion evaluation of hypoxia using optimal imaging tools.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Korea. This study was conducted in accordance with the recommendations of the Declaration of Helsinki for biomedical research involving human subjects and the Guidelines for Good Clinical Practice (ClinicalTrial.gov NCT02433639). Participants provided written informed consent before enrollment.
Author Contributions
Conceived and designed the analysis: Cheon GJ, Oh DY.
Collected the data: Lee KH, Cheon GJ, Oh DY.
Contributed data or analysis tools: Yoon J, Kang SY, Cheon GJ.
Performed the analysis: Yoon J, Oh DY.
Wrote the paper: Yoon J, Oh DY.
Conflicts of Interest
Author DYO: Research grant from AstraZeneca, Novartis, Array, Eli Lilly, Green Cross. Consultant/Advisory board for AstraZeneca, Novartis, Genentech/Roche, Merck Serano, Bayer, Taiho, ASLAN. Author JSY, SYK, KHL and GJC declare that they do not have any conflicts of interest.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1072 & HI18C1916). We thank all the investigators, and research staffs who supported this trial, the patients who participated in the trial, and their families.