Patterns of Failure after Postoperative Radiation Therapy for Endometrial Carcinoma
Article information
Abstract
Purpose
We tried to investigate the outcome and patterns of failure of endometrial cancer patients who were treated with surgery and postoperative radiation therapy (RT).
Materials and Methods
Eighty-three patients with endometrial cancer who received postoperative RT between May 1979 and August 2000 were included in this retrospective study. Forty-one patients received total abdominal hysterectomy, 41 patients received Wertheim's operation and 1 underwent vaginal hysterectomy. Pelvic lymph node dissection or pelvic lymph node sampling was done in 56 patients and peritoneal cytology was done in 35. All the patients were staged according to 1988 FIGO (International Federation of Gynecology and Obstetrics) staging system; 2 were stage IA, 23 were stage IB, 20 were stage IC, 4 were stage IIA, 5 were stage IIB, 9 were stage IIIA, 2 were stage IIIB and 18 were stage IIIC. The histologic diagnoses were adenocarcinoma in seventy-four patients (89%). The histologic grades were Grade 1, 2 and 3 in 21 (25%), 43 (52%) and 10 (12%) patients, respectively. All the patients received external beam RT (EBRT) with a median dose of 5,040 cGy (range: 4,500~5,075 cGy) to the whole pelvis. Five patients with pathologically confirmed paraaortic lymph node metastasis received 4500 cGy to the paraaortic lymph nodes. Fifteen patients received low-dose intracavitary brachytherapy after their EBRT. A total dose of 7,500~9,540 cGy (median dose: 8511) was prescribed to the vaginal surface.
Results
Overall, 11 patients (13%) experienced disease relapse: 4 with initial stage I or II disease and 7 with initial stage III disease. Among the 54 stage I or II patients, 1 (2%) relapsed in the pelvis only, 2 (4%) relapsed in the vagina and distant organs, and 1 (2%) relapsed in the paraaortic lymph nodes (PANs). Among the 29 stage III patients, 1 (3%) relapsed in the vagina. The most common sites of failure for the stage III patients were the peritoneum (3 patients, 10%), PANs (2 patients, 7%), and lung (2 patients, 7%). With a median follow-up period of 86 months, the overall survival (OS) and disease-free survival (DFS) rates at 5 years were 87% for both. The five-year DFS rate was 93%, 100% and 74% for the stage I, II and III patients, respectively. Three patients experienced severe radiation-related late complications: RTOG (Radiation Therapy Oncology Group) grade 3 radiation cystitis was seen in one patient, and grade 3 bowel obstruction was seen in two patients.
Conclusions
Postoperative RT was useful for controlling pelvic disease. The major patterns of failure for stage III patients were peritoneal seeding and distant metastasis. Selective use of whole abdominal radiotherapy or adjuvant chemotherapy may improve the therapeutic outcome of these patients.
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy in the United States and it's the fourth most common cancer of women (1). According to a recent report, the incidence of endometrial cancer is increasing annually in Korea (2). Its age-adjusted incidence rate has increased from 1.32 per 100,000 women in 1993 to 2.64 in 2002. Before 1997, this malady was most commonly seen in women of the seventh decade, but now it is most common in women of the sixth decade.
Most cases of endometrial cancer are diagnosed at FIGO stage I or II. In Korea, stage I comprised 63.6% of all the endometrial cancer cases in the year 2002 (2). The standard treatment for stage I, II and III endometrial cancer is surgery, and then postoperative RT is offered to the patients who are at high risk for disease recurrence. This retrospective study analyzed the outcome and patterns of failure of the patients who have received surgery and postoperative RT for endometrial cancer during the past 20 years at Seoul National University Hospital (SNUH).
MATERIALS AND METHODS
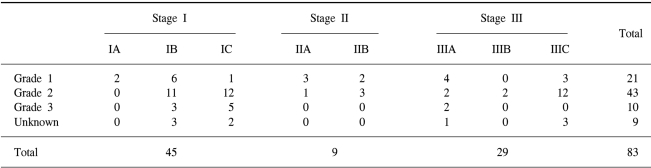
We reviewed the medical records of 83 patients who were suffering with stage I, II and III endometrial cancer and who were treated with surgery and postoperative RT at SNUH between May 1979 and August 2000. The inclusion criteria were as follows: (i) a total radiation dose ≥ 45 Gy, (ii) no previous or synchronous malignancy, (iii) no distant metastasis, and (iv) a follow-up period of more than 24 months. The median age of the patients was 56 years (range: 30~77). Information about the patients' menopause state, parity and combined medical illness (hypertension or diabetes mellitus) were not available for all patients. The clinical characteristics of the patients are listed in Table 1.
All the patients received hysterectomy. Forty-one patients received total abdominal hysterectomy, 41 received Wertheim's operation and 1 received vaginal hysterectomy. The histologic diagnoses were adenocarcinoma in 75 patients, adenosquamous cell carcinoma in 6 and adenoacanthoma in 2 patients. Fifty-four patients underwent pelvic lymph node dissection and two patients underwent pelvic lymph node sampling. Five patients underwent PAN sampling because they were suspected to have PAN metastasis on the preoperative computed tomography scan and this was pathologically confirmed in all cases. In 1989, standard surgical staging for endometrial cancer was introduced by the FIGO Committee on Gynecological Oncology (3). Therefore, peritoneal cytology examination was done for 18% (6/33) of our cases before 1990. But from 1990 on, 58% (29/50) of the cases were evaluated for peritoneal cytology. Four out of the 35 cases were positive for malignant cells.
All patients were staged according to the 1988 FIGO staging system. The proportion of FIGO Stage I, II and III was 54%, 11% and 35%. The distribution of the pathologic stage and grade is shown in Table 2.
Postoperative RT was administered within 3~8 weeks following surgery. All the patients received EBRT to the whole pelvis with using the four-field box technique. The superior border was specified at the L5-S1 disc and the inferior border at the bottom of the obturator foramina. The lateral borders are set 1.5 to 2.0 cm beyond the edge of the pelvic rim. The total dose was 4,500~5,075 cGy with a daily dose of 175~200 cGy. Five patients who had pathologically confirmed PAN metastasis received 4,500 cGy to the paraaortic lymph nodes with a daily fraction of 180 cGy. One stage IIIC patient with peritoneal seeding was treated with parallel opposed fields to the whole abdomen and pelvis with total dose of 3,000 cGy in 20 fractions. After whole abdominal irradiation, the paraaortic field and pelvis were boosted to a midplane dose of 4,620 cGy and 5,520 cGy, respectively. The fifteen patients treated before 1989 received a vaginal boost with brachytherapy following EBRT. The vaginal brachytherapy consisted of using Manchester ovoids. 3,000~4,500 cGy was delivered to the vaginal surface with a dose rate of 50~70 cGy/hr. The total dose prescribed to the vaginal surface was 7,500~9,540 cGy (median dose: 8,511 cGy).
Adjuvant chemotherapy was not routinely performed for the endometrial cancer patients; however, two patients of our study population received three cycles of postoperative combination chemotherapy with cisplatin, adriamycin (or epirubicin) and cyclophosphamide.
The overall survival (OS) and disease-free survival (DFS), as well as the freedom from pelvic recurrence (FPR) rates, were calculated using the Kaplan-Meier method (4). Log rank statistics were used for the comparing the prognostic variables (5). Multivariate analysis using Cox regression analysis was also performed (6). The acute and late radiation-related toxicities were evaluated using the RTOG criteria (7).
RESULTS
1) Patterns of failure
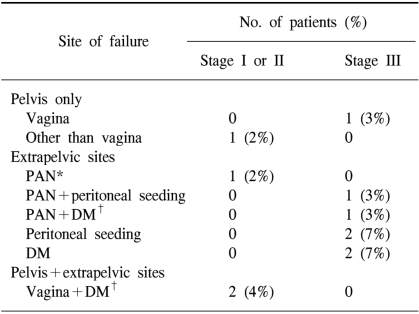
The patterns of failure are shown in Table 3. Altogether, eleven patients (13%) experienced disease recurrences. The median time to recurrences was 7 months (range: 3~91 months) from the date of surgery.
Among the fifty-four patients with stage I or II disease, one patient was found to have vaginal recurrence and liver metastasis simultaneously at 4 months after the surgery, and another patient was found to have supraclavicular lymph node metastasis and vaginal recurrence at 7 years after the surgery. There was an additional patient who relapsed at other intrapelvic site 8 months after the original surgery. The FPR rate at 5 years was 96% for all the stage I and II patients.
Among the fifty-four patients with stage I or II disease, there were twenty-one patients with stage IA or IB and grade 1 or 2 disease. Excluding these relatively low-risk patients, the 5-year FPR rate was 94%.
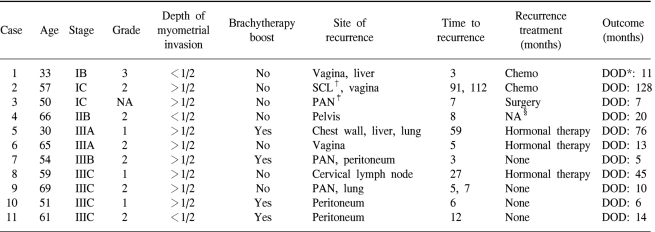
Among the twenty-nine stage III patients, one relapsed in the vagina. The most common sites of relapse were peritoneum (3 patients), PAN (2 patients) and lung (2 patients). Table 4 summarizes the clinicopathologic characteristics of the 11 recurrent cases.
No vaginal recurrence developed in the group of patients who received intracavitary vaginal cuff irradiation, including the two patients who demonstrated microscopically positive vaginal resection margins. On the other hand, three vaginal recurrences developed among the 68 patients who had not received brachytherapy.
2) The overall and disease-free survival rates
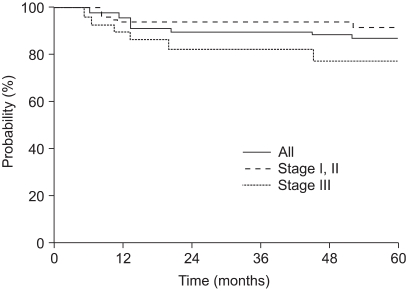
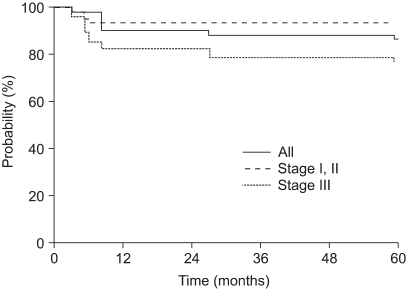
The median follow-up time was 86 months (range: 5~268 months). The OS and DFS rates at 5 years were both 87%.
The five-year OS, according to the stage, was 91%, 100% and 78% for stage I, II and III disease, respectively. The five-year DFS rate of stage I, II and III patients was 93%, 100% and 74%, respectively. The OS and DFS curves with using the Kaplan-Meier method are shown in Fig. 1 and 2. By the log rank test, the DFS of stage I and II patients was significantly higher than that of the stage III patients (94% vs. 74%, respectively, at 5 years, p=0.02). Yet the difference between the OS of stage I and II patients and that of stage III patients didn't reached statistical significance (92% vs. 78%, respectively, at 5 years, p=0.10).
3) Prognostic factors for OS and DFS
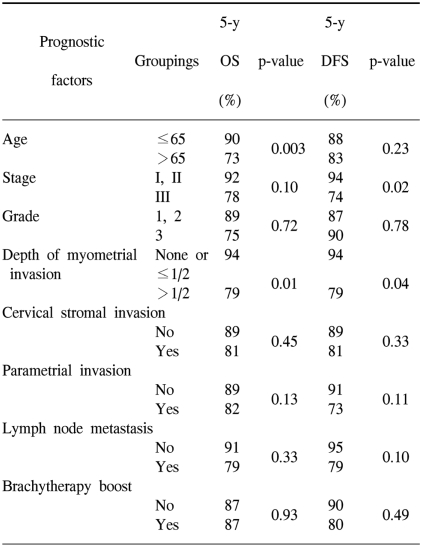
The prognostic variables evaluated for the univariate analysis included age (≤65 vs. >65), stage (stage I and II vs. stage III), grade (1 and 2 vs. 3), depth of myometrial invasion (≤1/2 of the myometrial thickness vs. >1/2 of the myometrial thickness), the presence of cervical stromal invasion, the presence of parametrial invasion, lymph node metastasis (no vs. yes) and the use of brachytherapy (no vs. yes). The univariate analysis of prognostic variables for OS and DFS is shown in Table 5. Age (p=0.003) and the depth of myometrial invasion (p=0.01) were significant factors for OS. For DFS, the stage (p=0.02) and depth of myometrial invasion (p=0.04) were statistically significant.
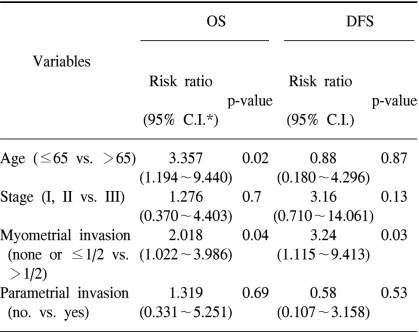
The variables included in the multivariate analysis were age, stage, depth of myometrial invasion and the presence of parametrial invasion (Table 6). In this analysis, an older age (>65 years old) and the presence of more than 1/2 depth of myometrial invasion were determined to be poor prognostic factors for OS (p=0.02 and 0.04, respectively). For DFS, the presence of more than 1/2 depth of myometrial invasion was the only significant variable for a poor DFS (p=0.03).
4) Complications
The acute toxicities were all RTOG Grade 0,1 or 2. Leukocytopenia (61%) was the most common hematologic toxicity. The frequent nonhematologic toxicities were abdominal pain (38%), stool frequency (35%) and nausea/vomiting (33%).
The overall RTOG grade 3 and 4 late complication rate was 4% (3 out of 83). There was one case of grade 3 radiation cystitis. Grade 3 small bowel obstruction developed in two patients, and one of these patients had received 45 Gy RT to the paraaortic field.
DISCUSSION
There have been controversies regarding the benefit of performing postoperative pelvic RT for FIGO stage I or II endometrial cancer. Yet the recently published large-scale randomized trials have demonstrated that postoperative pelvic RT decreases the incidence of pelvic recurrences, but it did not affects overall survival (8,9). In the GOG (Gynecologic Oncology Group) -99 study, 449 patients with stage IB to IIB endometrial adenocarcinoma with any grade were randomized after surgery to either the no additional therapy (NAT) group or the whole pelvic RT group. The estimated 2-year cumulative incidence of recurrence was 12% in the NAT arm and 3% in the RT arm (relative hazard (RH): 0.42; p=0.007). The estimated 4-year survival rate was 86% in the NAT arm and 92% for the RT arm. (RH: 0.86, p=0.557). In the PORTEC (Post Operative Radiation Therapy in Endometrial Carcinoma) study, 714 stage I patients, excluding those patients with stage IC, grade 3 or stage IB disease and grade 1 lesions, were randomized to the postoperative RT group or the no further treatment group. After long-term follow-up and a centralized pathology review, the 10-year locoregional relapse rates were 5% (for the RT group) and 14% (for the controls; p<0.0001), and the 10-year overall survival rates were 66% and 73%, respectively (p=0.09).
In the present study, stage I or II patients comprised about two-thirds of the study population and their 5-year OS rate was 92%. This overall survival rate is comparable with that of the RT arm in the randomized trials.
The five-year FPR and DFS rates of 96% and 94%, respectively, for the stage I and II patients of our study are better than those of the previous reports. Yet we included those patients with grade 1 or 2 disease and who also had invasion of less than 1/2 depth of the myometrial thickness. The five-year DFS and pelvic failure rates for these patients are reported to range from 90~95% and 0~5%, respectively, even without adjuvant RT (10~12). When excluding these 'low-risk' patients, the 5-year FPR and DFS rates of the stage I and II patients in our study were still over 90% (94% and 91%, respectively). We can suppose that the postoperative RT lowered the pelvic recurrence rate of the high-risk patients to the level of that of the low-risk patients.
Since 1988 at SNUH, postoperative RT hasn't been for the stage I endometrial cancer patients having grade 1 or 2 disease with invasion of <1/2 of the myometrial thickness. Recently, a vaginal brachytherapy boost has also not been indicated for endometrial cancer patients unless their surgical resection margins were positive for malignant cells.
The benefit of the brachytherapy vaginal cuff boost is controversial. Bliss and Cowie have retrospectively analyzed 91 endometrial cancer patients who received postoperative RT according to whether or not they received any additional intracavitary brachytherapy (13). Although the group receiving additional brachytherapy had more patients with poor prognostic factors, no vaginal vault recurrence was observed in that group. But a 10% (four out of forty patients) vaginal vault recurrence rate was observed in the group that received external beam therapy alone. Greven et al, have compared the 5-year pelvic control and DFS rates of the external beam RT alone group (Group A) with those of the external beam RT with brachytherapy boost group (Group B) (14). No difference was detected for the 5-year pelvic control and DFS rates between groups A and B.
In our study, only 18% of the patients received a brachytherapy boost and no vaginal recurrence was found in this group of patients. Although three vaginal vault recurrences were observed in the 68 patients who had not received brachytherapy boost, the differences of the FPR and DFS rates between the two groups were not statistically significant. Because the small number of patients in the group that received a brachytherapy boost, we cannot reach any conclusions regarding the benefit of brachytherapy boost.
For the stage III endometrial cancer patients in this study, the major patterns of failure were peritoneal seeding and distant metastases. PAN metastasis was frequently accompanied in these patients. Accordingly, the extrapelvic recurrence rate of the stage III patients was about 20%. More efforts need to be made to decrease the extrapelvic recurrence rate, and this may well prolong the survival of this patient group.
The GOG (Gynecologists Oncology Group) has recently reported the outcome of performing adjuvant whole abdominal RT in the patients with stage III and IV endometrial cancer (15). Of the 180 evaluable patients who entered that study, 103 had high-risk histology, i.e., either papillary serous or clear cell cancers. They found that all the patients with gross residual disease after surgery eventually failed, even with whole abdominal RT, and this was regardless of the histologic type. They concluded that whole abdominal irradiation may improve the outcome of the patients with completely resected disease, but in their study, grade 3 and 4 gastrointestinal toxicity was observed in 11% and 4% of patients, respectively. Considering its higher risk of causing gastrointestinal complications compared with whole pelvis RT, further investigations for selecting the patients who may benefit from whole abdominal RT is required.
There are still limited published data on the potential benefit of administering adjuvant chemotherapy combined with adjuvant RT. The phase II trials have shown a response to chemotherapy for unresectable, recurrent or metastatic endometrial cancer. Doxorubicin, cisplatin and paclitaxel have all been shown to have activity as a single agent, with response rates of 20~35% (16~19). RTOG reported the preliminary analysis of postoperative RT combined with cisplatin/paclitaxel chemotherapy for the patients with high-risk endometrial cancer (20). 66% of the eligible patients had stage lll disease. At the median follow-up of 28.7 months, the grade 3 and 4 chronic toxicities were 16% and 2%, respectively. The pelvic recurrence, regional recurrence, distant recurrence, DFS and OS rates at 24 months were 2%, 3%, 17%, 83% and 90%, respectively.
A phase III study (GOG122) randomized the patients with stage III and IV endometrial cancer to the whole abdominal irradiation group versus the chemotherapy with doxorubicin and cisplatin group. This study closed in February 2000 and its long-term results are being awaited. It will suggest some therapeutic guidelines for stage III and IV endometrial cancer.
Older age was a poor prognostic factor for OS, but not for DFS, in our study. This result is consistent with the result of a previous large cohort study (21). Deep myometrial invasion was the only significant prognostic factor for DFS in our study, but other pathologic factors such as stage, histologic grade, the peritoneal cytology, adnexal invasion, cervical extension, lymph node metastasis, the histologic type and lymph-vascular space invasion have been reported to be important prognostic factors in many other studies (22~25). The efforts to identify the risk factors for recurrences may be helpful in establishing better treatment strategies.
CONCLUSIONS
Postoperative RT was useful for controlling pelvic disease in this study. The major patterns of failure for stage III patients were peritoneal seeding and distant metastasis. Selective use of a vaginal brachytherapy boost or whole abdominal RT may decrease the rate of intrapelvic or extrapelvic disease recurrence. Furthermore, the recent investigations conducted on adjuvant chemotherapy in combination with radiotherapy are anticipated to shed new light on how to improve the therapeutic outcome of the endometrial cancer patients with high risk factors.