Occludin Expression in Brain Tumors and its Relevance to Peritumoral Edema and Survival
Article information
Abstract
Purpose
Peritumoral brain edema (PTBE) is a serious causative factor that contributes the morbidity or mortality of brain tumors. The development of PTBE is influenced by many factors, including such tight junction proteins as occludin. We evaluated the PTBE volume and survival time with respect to the occludin expression in various pathological types of brain tumors.
Materials and Methods
Fresh-frozen specimens from sixty patients who had brain tumors were obtained during surgery and the tumors were confirmed pathologically. The occludin expression was investigated by Western blot analysis. The PTBE volume was measured by using preoperative magnetic resonance (MR) imaging, and the survival time in each patient was estimated retrospectively.
Results
Occludin was detected in 41 (68.3%) of the cases with brain tumors and it was not expressed in the other 19 (31.7%) cases. Although the lowest expression was revealed in high-grade gliomas, its expression was variable according to the pathology of the brain tumors (p>0.05). The difference of PTBE volume between occludin-positive and negative brain tumors was statistically significant (2072.46±328.73 mm3 vs. 7452.42±1504.19 mm3, respectively, p=0.002). The mean survival time was longer in the occludin-positive tumor group than in the occludin-negative group (38.63±1.57 months vs. 26.16±3.83 months, respectively; p=0.016).
Conclusions
This study suggests that the occludin expression is highly correlated to the development of PTBE in brain tumors and it might be a prognostic indicator for patient survival.
INTRODUCTION
Peritumoral brain edema (PTBE) affects intracranial pressure, and this is one of the most serious complications in the management of intracranial neoplasms. Moreover, the severity and variety of PTBE may limit operative exposure and this frequently increases the difficulties of intraoperative procedures (1).
Malignant brain tumors such as glioblastoma and metastatic brain tumors cause PTBE because microvessels leak fluid from their lumens into the brain parenchyma (2). These malignant tumor vessels often lack tight junctions that are normally present in cerebral microvessels, and this can interfere with the function of the adjacent brain tissue (3). Tight junctions are specialized membrane domains that seal the intercellular spaces and act as a permeability barrier between the luminal and interstitial compartments.
Tight junction proteins such as occludin, claudin and junctional adhesion molecules that bind across adjacent cell membranes to "glue" them together have recently been found. Occludin is the best-characterized transmembrane protein, and it is a functional component of tight junctions and its widely expressed by essentially all epithelial and endothelial tissues (4,5).
This study was performed to investigate the occludin expression according to the pathological types of brain tumors and to verify the association of the occludin expression with the PTBE volume and the survival time for patients with brain tumors.
MATERIALS AND METHODS
1) Patients and samples
Surgical specimens were obtained from sixty brain tumors patients who had undergone surgery between January 2001 and December 2002. All the brain tumor tissues were collected during craniotomies for their resection or during biopsy; the tissues were immediately stored in a deep freezer at -70℃ prior to experimental study. Histopathologic evaluation of the brain tumor tissues was performed by a neuropathologist and this was based on the World Health Organization Grade 4 tier classification of gliomas (6). Gliomas were classified as low (grades I~II) or high (grades III~IV) grade. All the tumors also categorized into benign or malignant tumors. The malignant tumors included high grade gliomas and metastatic tumors, and the remaining pathology of the brain tumors was defined as benign tumors.
After surgery, clinical follow-up data was obtained from patients at 1, 3, 6 and 12 months for the first year and at 6-month intervals thereafter. The data was reviewed (Ed note: the follow-up period wasn't reviewed.) on December 2004. The survival time was measured as the time from the date of the initial surgery to the date of death or the latest follow-up.
2) Measurement of the PTBE volume
The PTBE volume was measured on the preoperative magnetic resonance (MR) images. The greatest anteroposterior (a) and lateral (b) diameters of the hyperintense lesions, except for the tumor seen on the axial T2-weighted MR images, and the greatest height (c) of the hyperintense areas on the coronal T2-weighted MR images was assessed. The PTBE volume was calculated by the formula V (mm3) = 4/3×πabc.
3) Western blot analysis
The brain tumor samples that had been stored in the deep freezer were rinsed three times with phosphate-buffered saline (PBS) and then lysed with using a homogenization buffer that contained 0.1 M sodium phosphate buffer (pH 6.1), 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM 1,4-Dithio-DL-threitol (DTT), 0.1 mM phenylmethylsulphonylfluoride (PMSF) and 1 mM benzamidine. The homogenates were centrifuged for 15 min at 12,000 rpm at 4℃. Their supernatants were diluted by a mixed solution of 950µl Laemmli sample buffer [sodium dodecyl sulphate (SDS) reducing buffer: 62.5 mM Tris-HCl (pH 6.8), 20% Glycerol, 2% SDS and 5% β-Mercaptoethanol] and β-Mercaptoethanol 50µl at the ratio of 1:2 and then the supernatants were boiled for 5 min. After protein quantification was performed by using the DC Protein Assay (Bio-Rad, Hercules, CA), 15µl of the proteins were loaded on each lane and they were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on 10% acrylamide gel. A protein marker was used for the pre-stained SDS-PAGE standard (Bio-Rad Labs Ltd., Hemel Hemstead, Hertfordshire, UK). After electrophoresis, the gel was equilibrated with towbin buffer (25 mM Tris, 192 mM glycine and 20% methanol) for 60 min and the proteins were then transferred overnight onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The membranes were saturated for 1 h using 5% dry milk in TBS-T (10 mM Tris-buffer pH 7.5, 100 mM NaCl and 0.1% Tween 20) and they were incubated overnight with 1:1000 occludin monoclonal antibody (Zymed, San Francisco, CA) at 4℃. The membranes were then incubated for 1 h with a 1:2000 mouse anti-mouse IgG antibody conjugated to alkaline phosphatase (Zymed, San Francisco, CA) as the secondary antibody. The detection of proteins was followed with the ECL detection system (Amersham, Arlington Height, IL). The blots were washed and the bands were visualized by ECL plus (Amersham, Arlington Heights, IL) and autoradiography (Fig. 1).
4) Statistical analysis
The data were analyzed using SPSS 10.0 for windows (SPSS Inc, Chicago, IL). Statistical difference of the occludin expression in the benign and malignant brain tumors was analyzed by Fisher's exact test. Mann-Whitney's test was employed to compare the PTBE volume and survival time between the occludin positive and negative brain tumors.
According to the pathology of the brain tumors, the difference of occludin expression and PTBE volume were analyzed by the Kruskal-Wallis test. Correlation of survival time to the PTBE volume was done by Pearson's correlation test and this was depicted by a Kaplan-Meier survival curve. In addition, the survival time according to the occludin expression in brain tumors was analyzed by the Log-rank test. All the data was expressed as means±standard deviations. Differences with a p value of 0.05 or less were regarded as statistically significant.
RESULTS
1) Patient characteristics
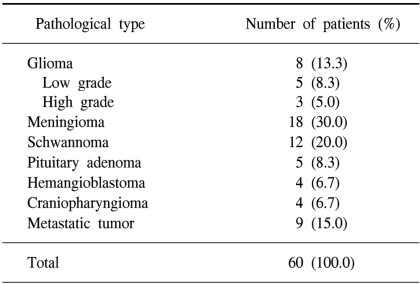
Sixty patients were enrolled in the study and their mean age was 44.9 years (range: 2~79 years). Twenty-six patients were male and 34 patients were female. This study group included 8 patients with glioma, 5 patients (8.3%) with low grade tumor, 3 patients (5.0%) with high grade tumor, 18 patients (30.0%) with meningiomas, 12 patients (20.0%) with schwannomas, 5 patients (8.3%) with pituitary adenomas, 4 patients (6.7%) with hemangioblastomas, 4 patients (6.7%) with craniopharyngiomas and 9 patients (15%) with metastatic tumors (Table 1).
2) Occludin expression
Occludin was detected in 41 (68.3%) cases with brain tumor (Fig. 1). However, the difference of occludin expression according to the pathological types was not statistically significant (p>0.05). A high expression rate of occludin was found in the hemangioblastomas (100%), pituitary adenomas (100%), and schwannomas (83.3%) (Fig. 2). 79% of the benign tumors expressed occuludin (38/48), whereas 25% (3/12) of malignant tumors expressed occuludin. This difference was significant statistically (p=0.009 by Fisher's exact test).
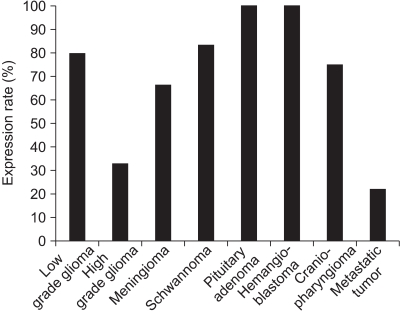
Bar graph demonstrating the expression rate of occludin according to pathological types of different brain tumors. The high expression rate of occludin is noted in hemangioblastoma and pituitary adenoma; however, its difference was not significant among the pathological types of brain tumor (p>0.05).
3) PTBE volume
The mean PTBE volume was 3776.12±612.21 mm3 in all tumors, 2,072.46±328.73 mm3 in the occludin-positive tumors, and 7,452.42±1,504.19 mm3 in the occludin-negative tumors (p=0.002 by Mann-Whitney test) (Fig. 3). PTBE volume according to the pathology of the brain tumors is shown in Fig. 4 and the PTBE volume was increased in cases of malignant tumors. Specifically, it measured as a high value of 1,074.00±2,566.59 mm3 in metastatic tumors, 7,285.67±2,469.37 mm3 in high grade gliomas, and 4,119.23±823.9 mm3 in meningiomas. However, the PTBE volume was relatively low as 179.00±113.21 mm3 in craniopharyngiomas and 134.00±45.56 mm3 in pituitary adenomas (p>0.05 by Kruskal-Wallis test) (Fig. 4).
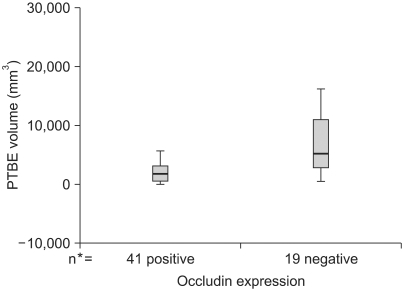
Graphs showing the peritumoral brain edema (PTBE) volume according to the occludin expression in brain tumors. A lower PTBE volume is noted in the positive group while a higher PTBE volume is evident in the negative group (p=0.002). *number of cases.
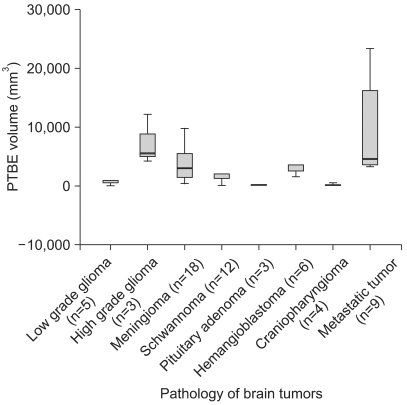
Graph demonstrating the PTBE volume according to the pathology of the various brain tumors. There are high PTBE volumes in the malignant brain tumors such as metastatic tumors and high grade gliomas. However, the differences among pathological types of brain tumor (p>0.05) are not significant. *number of cases.
4) Survival time
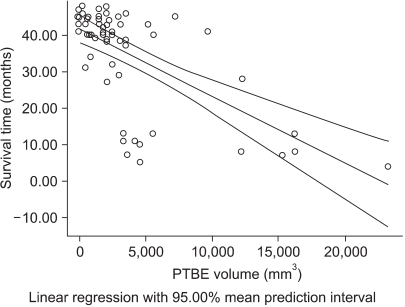
The difference of survival time between the occludin-positive tunors and occludin-negative tumors is shown in Fig. 5. In this study, the mean survival time was 34.68±1.77 months for all brain tumors, 38.63±1.57 months for the occludin-positive tumors and 26.16±3.83 months for the occludin-negative tumors (p=0.016 by Mann-Whitney test), respectively (Fig. 5). Survival time was inversely correlated to the PTBE volume (R-square=0.40, p=0.001 by Pearson's correlation test) (Fig. 6).

Graph demonstrating the result of comparison of the survival time of positive occludin expression vs. negative occludin expression groups of brain tumors. The positive-occludin expression group shows significantly longer survival time in contrast to the negative-occludin expression group (p=0.016). *number of cases.
DISCUSSION
The present study has shown that occludin was expressed in various brain tumors and the PTBE volume was lower in the occludin-positive group. The PTBE volume was greater for the malignant brain tumors such as the metastatic tumors and high grade gliomas, and the occludin-positive tumor group tended to have a significantly longer survival time in contrast to the occludin-negative tumor group. Additionally, this study demonstrated an inverse relationship between survival time and the PTBE volume.
Brain edema is defined as a net increase of the water content of the brain parenchyma. Redistribution of water between the intracellular and intercellular compartments, or an increase in cerebral blood or cerebrospinal fluid volume does not, therefore, constitute brain edema. All aggressive brain tumors such as malignant gliomas and metastatic tumors, and many benign tumors such as meningiomas produce brain edema regardless of the origin of their cell type (7). Because the skull is non-compliant, brain edema causes increased intracranial pressure (ICP) that potentially leads to brain ischemia, herniation and death. The lethal effect of edema is illustrated by a 10-fold reduction in operative mortality of brain tumors in the 1960s, which resulted from treating brain edema with corticosteroids (8).
Occludin bands have been variously identified as splice variants and the phosphorylated forms (9). The hyperphosphorylated form of occludin appears to play an important role in the functional assembly of tight junctions (10). The 55 kDa occludin was shown to be present in non-neoplastic brain and there was a significant inverse correlation between the occludin expression and increasing malignancy (11).
This study demonstrated a high level of occludin expression in brain tumors, including hemangioblastoma and pituitary adenoma. But its difference according to the pathological type did not reach to statistical significance.
Electron microscopy revealed opening of tight junctions of the microvessels in high grade astrocytoma. The expression of tight junction protein occludin was reduced in these microvessels and this reduction was inversely correlated with the degree of cerbral edema. Normal astrocytes secrete factors that induce barrier properties in endothelial cells, whereas high grade astrocytomas secrete vascular endothelial growth factor (VEGF), which stimulates angiogenesis, down-regulates occludin and increases endothelial cell permeability.
Ultrastructural studies of human glioma microvessels have revealed 'open' interendothelial cell tight junctions (12). Tight junctions not only separate distinct physiological compartments, but they also confer selectivity to the transepithelial flux of molecules and ions through the intercellular spaces between the epithelial cells, the so-called paracellular pathway (13). Occludin and claudin are transmembrane proteins of tight junctions that probably participate in sealing the paracellular space (4). Occludin was the first identified transmembrane protein of tight junctions (5). Thus, the expression level of occludin directly affects the strength of the junctional seal in a manner that appears to depend on the genetic background of the analyzed cells. A possible explanation of this is that occludin is a regulatory component of the tight junction seal (14). A recent study reported that occludin also participated in the regulation of signaling processes that control epithelial transformation (15). Claudins were more recently discovered and they are a family of tight junction proteins that weigh 22~24 kDa (4). Claudin-1 and -5 together with occludin are the most important constituents of the blood brain barrier tight junctions (16). The expression of tight junction protein claudin-1 was lost in the majority of tumor microvessels, whereas claudin-5 and occludin were significantly downregulated only in hyperplastic vessels (16).
Recent studies have demonstrated the expression of VEGF in meningioma tissues, suggesting that VEGF plays an important role in both meningioma vascularity and peritumoral edema (17). Thus, VEGF may be important in the pathogenesis of brain tumor edema by inducing a reduction in the expression of endothelial tight junction proteins, including occludin (18,19). If an investigation on the co-expression of VEGF and occludin was to be undertaken in the same study group of brain tumor patients, the results will be interesting.
Dexamethasone is the mainstay of treatment for brain tumor edema (8). However, it is unlikely that dexamethasone has any effect on the 55-kDa occludin expression in non-neoplastic human brain microvessels (11). Treatment of primary human astrocytes with the cytokine interleukin-1β (IL-1β) caused downregulation of occludin, but this caused no change in the expression of zonula occludens (ZO) proteins ZO-1 and ZO-2. This suggested that in pathological conditions of the human CNS, an elevated IL-1β expression fundamentally altered astrocyte to astrocyte connectivity (20).
Our study has some limitations. It included various pathologic tumor types and tumor of different origins, including intraaxial vs extraaxial brain tumors. These biases may have influenced the results of this study.
According to this study, the occludin-positive expression group tended to have a significantly longer survival time comparable to an occludin-negative expression group. There is an inverse relationship between survival time and the PTBE volume. Further studies should be performed to understand the relationship among PTBE, survival time and occludin expression to decrease the morbidity and mortality and to improve patients' outcomes from the PTBE-related complications of brain tumors. Further, the correlation of many prognostic factors that can be associated with the expression of occludin should be verified in future studies.
CONCLUSIONS
This study suggests that the expression of occludin is closely correlated to the development of PTBE in patients with brain tumors and it might be a prognostic indicator for survival of these patients. A thorough understanding of tight junction protein such as occludin in brain tumor microvessels may allow the development of novel drugs that selectively open or close the blood-tumor barrier.