Adjuvant Chemotherapy in Microsatellite Instability–High Gastric Cancer
Article information
Abstract
Purpose
Microsatellite instability (MSI) status may affect the efficacy of adjuvant chemotherapy in gastric cancer. In this study, the clinical characteristics of MSI-high (MSI-H) gastric cancer and the predictive value of MSI-H for adjuvant chemotherapy in large cohorts of gastric cancer patients were evaluated.
Material and Methods
This study consisted of two cohorts. Cohort 1 included gastric cancer patients who received curative resection with pathologic stage IB-IIIC. Cohort 2 included patients with MSI-H gastric cancer who received curative resection with pathologic stage II/III. MSI was examined using two mononucleotide markers and three dinucleotide markers.
Results
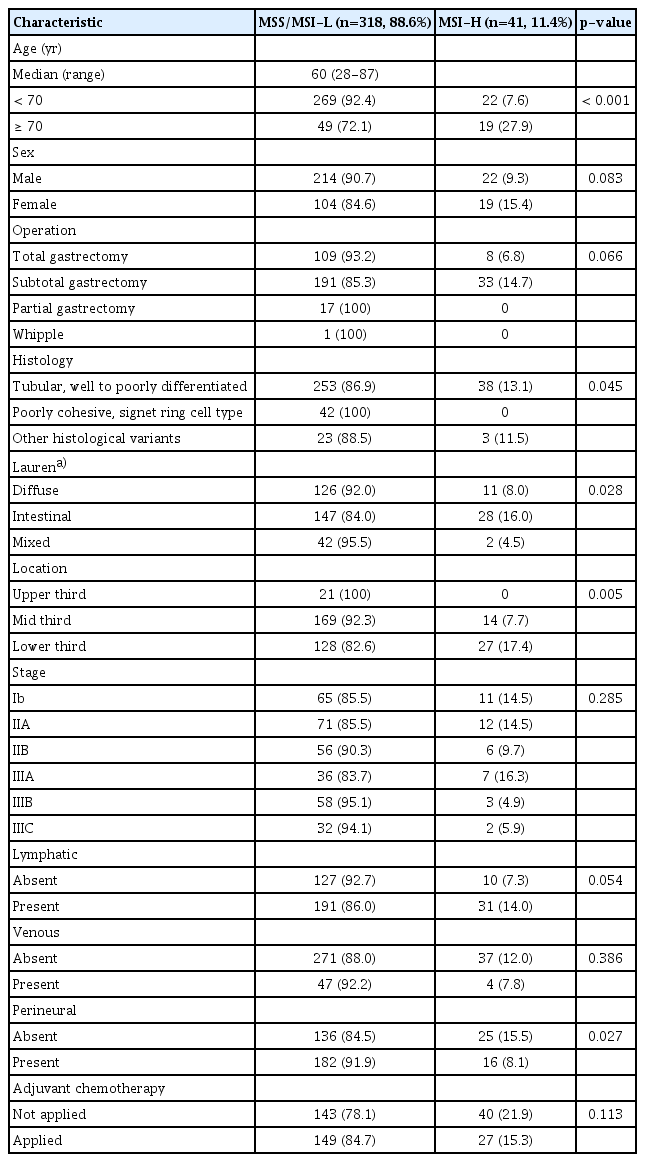
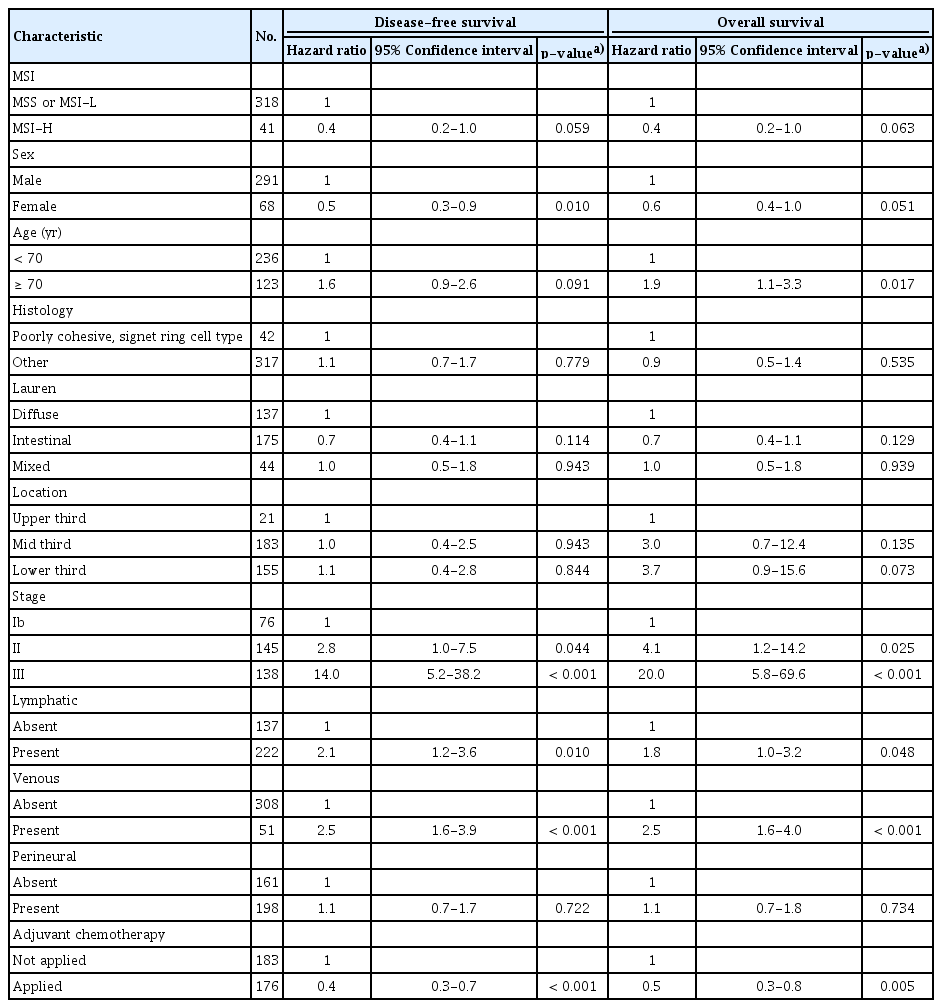
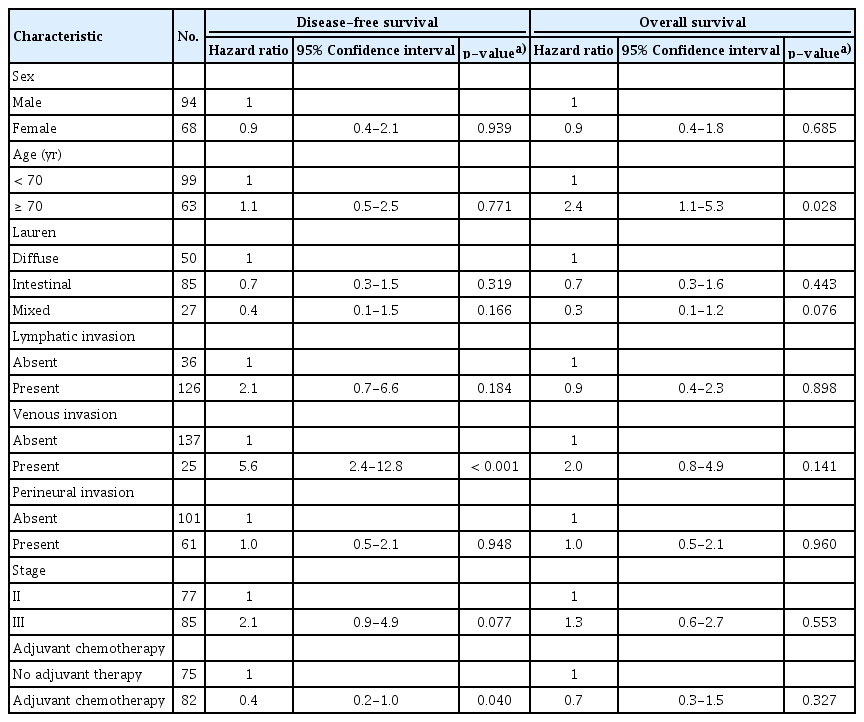
Of 359 patients (cohort 1), 41 patients (11.4%) had MSI-H. MSI-H tumors were more frequently identified in older patients (p < 0.001), other histology than poorly cohesive, signet ring cell type (p=0.005), intestinal type (p=0.028), lower third tumor location (p=0.005), and absent perineural invasion (p=0.027). MSI-H status has a tendency of better disease-free survival (DFS) and overall survival (OS) in multivariable analyses (hazard ratio [HR], 0.4; p=0.059 and HR, 0.4; p=0.063, respectively). In the analysis of 162 MSI-H patients (cohort 2), adjuvant chemotherapy showed a significant benefit with respect to longer DFS and OS (p=0.047 and p=0.043, respectively). In multivariable analysis, adjuvant chemotherapy improved DFS (HR, 0.4; p=0.040).
Conclusion
MSI-H gastric cancer had distinct clinicopathologic findings. Even in MSI-H gastric cancer of retrospective cohort, adjuvant chemotherapy could show a survival benefit, which was in contrast to previous prospective studies and should be investigated in a further prospective trial.
Introduction
Microsatellite instability (MSI) is characterized as the increased rate of uncorrected replication errors at the simple repeat sequence caused by a DNA mismatch repair gene (MMR) defect [1,2]. MSI-high (MSI-H) results in accelerated mutations in oncogenes and tumor suppressor genes and a phenotype of hypermutational status [3,4]. Tumor-specific neopeptides may be generated during MSI-H carcinogenesis. A protective role of lymphocytes against MSI-H colorectal cancer that prevents tumor metastasis was reported [5]. Because of the immunologic aspect of MSI status, it was recently highlighted as a predictive marker in immunotherapy. MSI-H tumors have been shown to benefit from immunotherapy, and anti–programmed death-1 antibody (pembrolizumab) has finally been approved by the U.S. Food and Drug Administration for the treatment of MSI-H tumors regardless of the tumor type [6,7].
In an adjuvant setting, MSI-H could be a prognostic and predictive marker. In colorectal cancer, MSI-H tumor showed better prognosis than microsatellite stable (MSS)/MSI-low (MSI-L) tumors [8,9]. Patients with MSI-H colorectal cancer did not benefit from adjuvant chemotherapy. Particularly, adjuvant chemotherapy with 5-fluorouracil alone in patients with stage II colorectal cancer may be even worse than no adjuvant chemotherapy [8]. Therefore, adjuvant chemotherapy with 5-fluorouracil alone in stage II colorectal cancer patients is not recommended by several guidelines [10,11].
In gastric cancer, adjuvant chemotherapy with capecitabine plus oxaliplatin or S1 has been proven to prolong survival after D2 resection of stage II/III gastric cancer in CLASSIC and ACTS-GC study [12,13]. MSI-H status is relatively common in gastric cancer and occurs in approximately 9% of surgically resected gastric cancer [4,14-16]. In the CLASSIC trial, compared with the overall positive results, patients with MSI-H gastric cancer did not experience any survival benefit from adjuvant chemotherapy [13,15]. Similarly, in the MAGIC trial, which evaluated the role of perioperative chemotherapy for resectable gastric cancer, MSI-H status had an improved prognosis in the surgery-alone treatment arm, but a worse survival outcome in the chemotherapy-plus-surgery arm compared with an MSS/MSI-L [17,18]. A recently published paper of pooled individual patient data from four large randomized clinical trials conducted in patients with resectable gastric cancer (MAGIC [18], CLASSIC [13], ARTIST [19] which evaluated the concurrent irradiation with adjuvant chemotherapy [capecitabine plus cisplatin], and ITACA-S [20] which evaluated an intensified combination chemotherapy schedule [fluorouracil plus leucovorin plus irinotecan followed by cisplatin plus docetaxel] compared with single-agent chemotherapy [fluorouracil plus leucovorin]) showed that patients with MSI-L/MSS gastric cancer benefited from chemotherapy plus surgery, but those with MSI-H gastric cancer did not [16]. However, in the CLASSIC and MAGIC trial, only 40 and 20 patients had MSI-H tumors. In a pooled analysis of four clinical trials, 121 patients had MSI-H tumors, and just 33 patients with MSI-H tumor who received surgery alone were included in the control group. Therefore, despite these results from randomized clinical trials and pooled analysis, there is limited statistical power for the use of MSI/MMR deficiency testing as a predictive marker for adjuvant chemotherapy in patients with curatively resected gastric cancer.
Therefore, we evaluated the predictive value of MSI-H tumor for the benefit of adjuvant chemotherapy in large cohorts of gastric cancer patients. The clinical characteristics of MSI-H gastric cancer were also evaluated.
Materials and Methods
1. Patients
This study consisted of two cohorts. In cohort 1, the clinical features of MSI-H compared with MSS/MSI-L were analyzed. Cohort 1 included gastric cancer patients who received curative resection with pathologic stage IB-IIIC from February 2005 to January 2006 at Seoul National University Hospital (SNUH). Cohort 2 was used for the analysis of the efficacy of adjuvant chemotherapy in MSI-H gastric cancer. Cohort 2 included patients with MSI-H gastric cancer who received curative resection with pathologic stage II/III from January 2007 to February 2012 at Seoul National University Bundang Hospital (SNUBH) and from December 2004 to June 2012 at SNUH. MSI-H patients in cohort 1 were included in cohort 2. Clinical data were retrieved from the medical records of patients. The American Joint Committee on Cancer (AJCC) 7th edition was used.
2. Test for MSI
Genomic DNA of the formalin-fixed gastric cancer tissues was extracted using standard proteinase-K digestion and a phenol/chloroform procedure. MSI was examined using two mononucleotide markers: BAT 25 (located 4q12-13 KIT gene, intron 16, T25 repeat) and BAT 26 (located 2p22-21, hMSH2 gene, exon5, A26 repeat) and three dinucleotide markers: DS123 (located 2p 16.3, CA repeat), D5S346 (located 5q22.2, CA repeat), and D17S250 (located 17q12, CA repeat). Polymerase chain reaction (PCR) reactions were conducted in 10 µL reaction volumes with fluorescent dye 50-end labeled primers. PCR products were denatured in formamide for 2 minutes at 95°C and electrophoresed on denaturing 8% polyacrylamide sequencing gels. MSI status was analyzed using GeneScan software in an ABI 3100 sequencer (Foster City, CA). Tissue samples that exhibited abnormal band patterns were considered to indicate MSI. According to the number of markers displaying instability of each tumor, the tumors were divided into MSI-H, MSI-L, and MSS. MSI-H indicated instability in two or more of five markers. MSI-L indicated instability in one of five markers. MSS indicated there was no instability in the five markers [1].
3. Statistical analysis
The differences in clinicopathologic findings according to MSI status were evaluated using chi-square analysis. Disease-free survival (DFS) was defined as the duration between the surgical operation and disease relapse, any cause of death before disease relapse, or the last follow-up. The event for DFS was defined as relapse and any cause of death. Overall survival (OS) was measured from the surgical operation to the last follow-up or any cause of death. The event for OS was defined as any cause of death. The Kaplan-Meier method was used for survival analysis with the log-rank test. The Cox proportional hazards regression model was used to calculate hazard ratio (HR) in univariable and multivariable analysis. All statistical analyses were performed using SPSS Statistics ver. 25 (IBM Corp., Armonk, NY).
4. Ethical statement
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and the national and international guidelines. This study was approved by the institutional review board at SNUBH (B-1207-164-107) and SNUH (H-1208-100-422) and acquired a waiver of informed consent.
Results
1. Characteristics of patients with MSI-H gastric cancer compared with MSS/MSI-L gastric cancer (cohort 1)
Cohort 1 was analyzed to evaluate the characteristics of MSI-H gastric cancer patients who received curative gastrectomy. A total of 731 patients with gastric cancer received the curative operation with D2 dissection. Among these patients, 366 patients with stage IA were excluded. MSI data were available in 359 of 365 patients with stages IB-IIIC. Cohort 1 consisted of these 359 patients (Table 1). MSI-H status was seen in 41 patients (11.4%) and was associated with older age (27.9% vs. 7.6%, p < 0.001), other histology than poorly cohesive, signet ring cell type (p=0.045), Lauren’s intestinal type (16% vs. 8.0% of disuse type, p=0.028), and lower third tumor location (17.4% vs. 7.7% of mid-third location, p=0.005). In contrast, there were no patients who had MSI-H tumors with poorly cohesive, signet ring cell type (0/42, 0.0%). There was no significant correlation according to stage (p=0.285), lymphatic invasion (p=0.054), or venous invasion (p=0.386), but MSI-H was identified more frequently in absent perineural invasion (p=0.027).
2. Survival of patients with MSI-H gastric cancer compared with MSS/MSI-L gastric cancer (cohort 1)
In cohort 1, the median follow-up duration was 71.1 months after surgery. Univariable analysis between clinicopathologic factors and survival was done (S1 Table). Patients with MSI-H gastric cancer had better 5-year DFS rate (83.2% vs. 65.5%, respectively; p=0.070) (Fig. 1A), and 5-year OS rate (84.4% vs. 71.7%, respectively; p=0.091) (Fig. 1B) than those with MSS/MSI-L, although there was not significant. In the multivariable analysis with MSI status, sex, age, World Health Organization histology, Lauren classification, tumor location, lymphatic invasion, venous invasion, perineural invasion, and the use of adjuvant chemotherapy, MSI-H status showed an better survival although there was not statistical significance (for DFS: HR, 0.4; 95% confidence interval [CI], 0.2 to 1.0; p=0.059; for OS: HR, 0.4; 95% CI, 0.2 to 1.0; p=0.063) (Table 2).
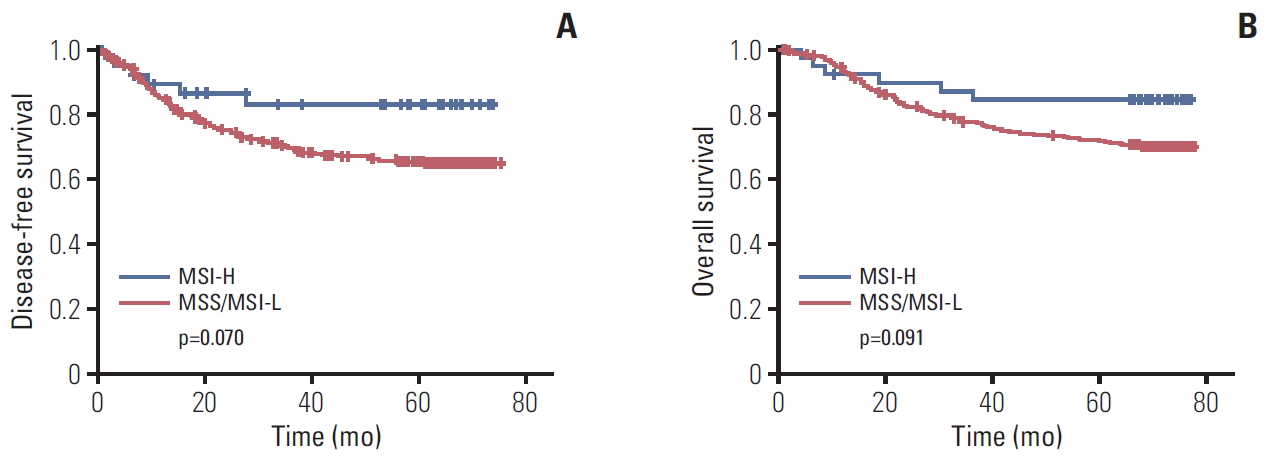
Disease-free survival (A) and overall survival (B) according to microsatellite instability (MSI) status. p-value calculated by a Kaplan-Meier method. MSI-H, MSI-high; MSI-L, MSI-low; MSS, microsatellite stable.
In terms of the role of adjuvant chemotherapy in MSS/MSI-L tumors, patients with adjuvant chemotherapy had the prolonged survival (for DFS: p=0.176 and p < 0.001; for OS: p=0.225 and p < 0.001 in stage II and stage III, respectively). However, in MSI-H tumors, patients with adjuvant chemotherapy did not show the prolonged survival because of small sample size (for DFS: p=0.439 and p=0.836; for OS: p=0.439 and p=0.933 in 18 patients with stage II and 12 stage III, respectively).
3. Characteristics of MSI-H gastric cancer patients (cohort 2)
We next evaluated the efficacy of adjuvant chemotherapy in more MSI-H gastric cancer patients who received a curative gastrectomy (cohort 2). In total, 5,983 patients were screened who received a gastrectomy and an MSI test. Of these 5,983 patients, 578 patients (9.7%) were confirmed as MSI-H. Finally, 162 patients who underwent R0 resection, were diagnosed with pathologic stage II/III, and were a candidate for adjuvant chemotherapy were included in cohort 2 (S2 Fig.). The baseline characteristics of these patients are shown in Table 3. In this cohort, 38.9% of patients were over 70 years old, and 58% were male. Tumor location of the lower third was observed in 62.3%. Intestinal type was found in 52.5%. All patients had MSI-H gastric cancer, and 69.8% of patients showed instability in all five MSI markers. Pathologic stage II and stage III were identified in 77 (47.5%) and 85 (52.5%), respectively. Lymph invasion, vascular invasion, and perivascular invasion were identified in 77.8%, 15.4%, and 37.7%, respectively. In addition, 75 patients (46.3%) were not treated with adjuvant chemotherapy. Fluoropyrimidine treatment alone, such as S1 or uracil and tegafur/leucovorin, was used in 42 patients (25.9%), whereas 40 patients (24.7%) were treated with fluoropyrimidine plus platinum, including 5-fluorouracil plus cisplatin or capecitabine plus oxaliplatin. There was no difference in the use of adjuvant chemotherapy by stage, but the application of adjuvant chemotherapy decreased significantly with age (p=0.521 and p < 0.001). Of the patients who were ≥ 80 years old, 81.8% did not receive chemotherapy. In contrast, 82.4% of the patients under 60 years old were treated with adjuvant chemotherapy, and 51.0% received combination therapy with fluoropyrimidine and platinum (p < 0.001) (S3 Table).
4. Survival according to adjuvant chemotherapy in MSI-H gastric cancer patients (cohort 2)
In cohort 2, the median follow-up duration was 87.9 months after surgery. Median DFS and OS of all patients were not reached. The result of univariable analysis between clinicopathologic factors and survival was shown in S4 Table. MSI-H patients treated with adjuvant chemotherapy showed longer DFS and OS than patients without chemotherapy (p=0.047 and p=0.043, respectively) (Fig. 2A and B). In MSI-H patients with stage II, this benefit of adjuvant chemotherapy was observed (p=0.001 in DFS, p=0.001 in OS) but not in MSI-H patients with stage III (p=0.867 in DFS, p=0.840 in OS). In patients who received fluoropyrimidine alone, the 5-year DFS and OS rates were 87.0% and 94.8%, respectively, which were higher than in the no adjuvant chemotherapy group (5-year DFS rate, 72.9%; p=0.044 [Bonferroni-corrected]; 5-year OS rate, 78.3%; p=0.022 [Bonferroni-corrected]) (Fig. 2C and D). In the fluoropyrimidine plus platinum group, the 5-year DFS and OS rates were 72.4% and 89.5%, respectively, which were not significantly different from the no adjuvant chemotherapy (p=0.880 [Bonferroni-corrected] and p=0.956 [Bonferroni-corrected], respectively). In addition, multivariable analysis with clinically significant factors, such as sex, age, stage, lymphatic invasion, venous invasion, perineural invasion, Lauren classification, and adjuvant chemotherapy, indicated that adjuvant chemotherapy in MSI-H gastric cancer patients was a significant independent prognostic factor for DFS (HR, 0.4; 95% CI, 0.2 to 1.0; p=0.040) (Table 4).
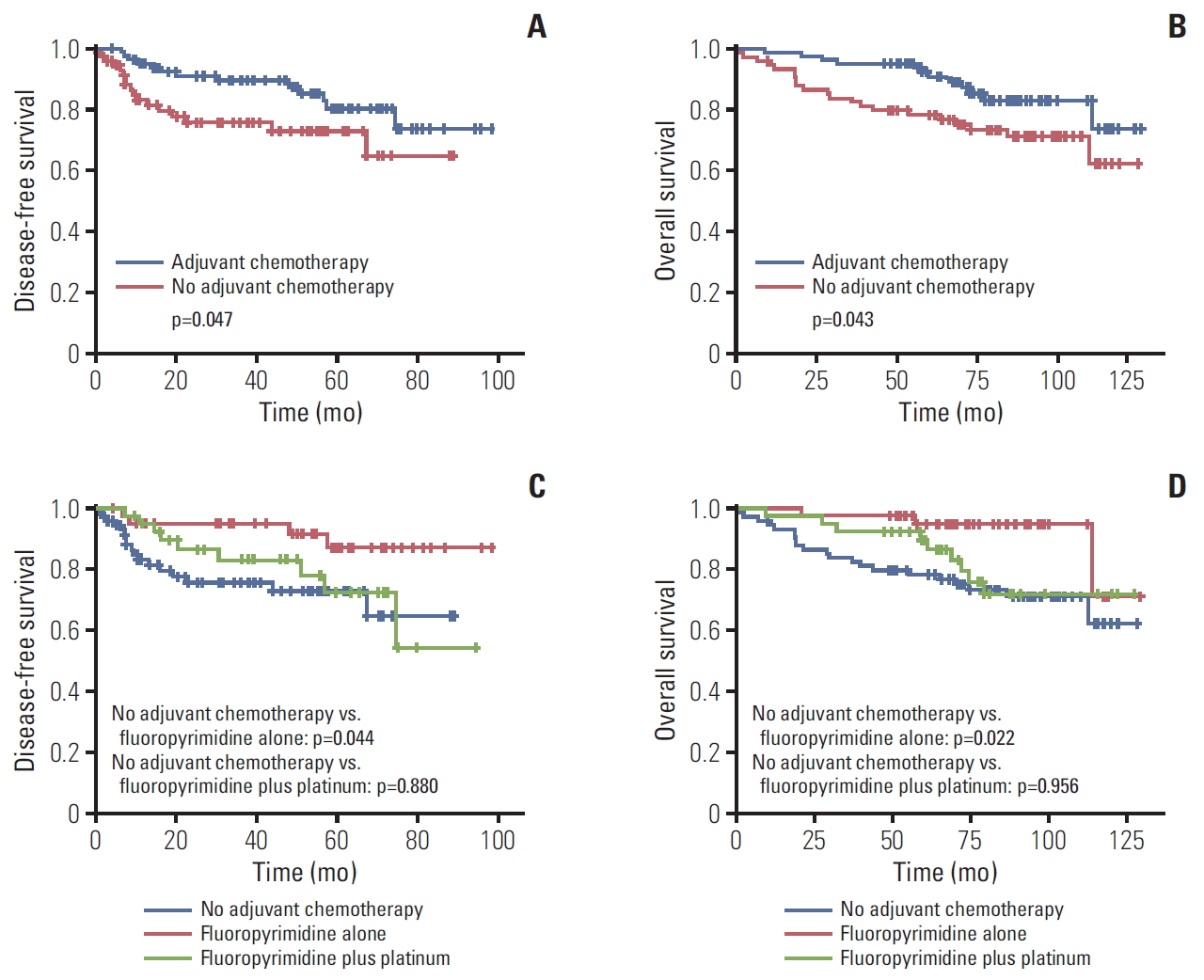
Survival according to adjuvant chemotherapy in microsatellite instability–high gastric cancer. Bonferroni-corrected p-values, calculated by a Kaplan-Meier method. (A) Disease-free survival according to adjuvant chemotherapy. (B) Overall survival according to adjuvant chemotherapy. (C) Disease-free survival according to chemotherapy regimen. (D) Overall survival according to chemotherapy regimen.
Discussion
In this study, the clinical features and predictive role of MSI-H for adjuvant chemotherapy were evaluated in curatively resected gastric cancer. MSI-H gastric cancer had a tendency of better prognosis than MSS/MSI-L after curative resection. In terms of the efficacy of adjuvant chemotherapy in MSI-H tumors, patients who received adjuvant chemotherapy could experience longer survival than those without adjuvant chemotherapy. Particularly, even adjuvant chemotherapy with fluoropyrimidine alone showed better survival than without adjuvant chemotherapy.
The clinical characteristics of MSI-H in curatively resected gastric cancer were distinctive from MSS/MSI-L. MSI-H tumor was diagnosed more frequently in older patients. In addition, MSI-H tumor was more common in patients with the intestinal type, other histology than poorly cohesive, signet ring cell type, and lower third tumor location. These findings were in accordance with previous studies [14,16]. In this study, MSI-H tumor was associated with present lymphatic invasion and absent perineural invasion. MSI was not correlated with early-stage cancers. This could be attributed to the exclusion of IA stage patients. In a previous study that included stage IA patients, MSI was observed more frequently in stage I (64.1%; T1, 44.1%; N0, 63.5%). Among stage II/III that were candidates for adjuvant chemotherapy, MSI-H was not observed frequently in earlier stage tumors [16].
MSI-H may be identified in just 7%-9% of resectable gastric cancers [14,16]. In the early stage that is not a candidate for adjuvant chemotherapy, MSI-H might be more frequently identified. Therefore, evaluation of the efficacy of adjuvant chemotherapy in MSI-H should be limited due to the small sample size. In previous studies related to the effects of adju-vant chemotherapy in MSI-H gastric cancer, only 40 and 20 MSI-H patients from the CLASSIC and the MAGIC study were included [15,17]. Just 33 MSI-H patients who received surgery alone and 88 patients with MSI-H tumor who received preoperative or adjuvant chemotherapy were included in the pooled analysis of four randomized trials [16]. In a retrospective study of a large cohort of 1,990 patients, just 54 patients with stage II/III were included for analysis of 5-fluorouracil adjuvant chemotherapy [14]. These studies demonstrated that MSI-H tumors did not benefit from adjuvant chemotherapy compared with MSS/MSI-L. However, due to small sample sizes, the use of these results to guide the application of adjuvant chemotherapy according to MSI status is limited. Although our study had a limitation due to its retrospective design, the population that was analyzed included 162 pathologic stage II/II patients with MSI-H tumor who were a candidate for adjuvant chemotherapy. This sample size was much larger than in previous studies. In contrast to previous studies, adjuvant chemotherapy could prolong the survival of patients, even in MSI-H gastric cancer. The efficacy of adjuvant chemotherapy in MSI-H patients could be an important clinical issue considering that MSI-H is more prevalent in older patients and those with an early stage of the disease. Therefore, this controversial result should be investigated in a further prospective study. Additionally, adjuvant chemotherapy with fluoropyrimidine alone showed a benefit in terms of survival, but fluoropyrimidine and platinum combination did not show better survival as an adjuvant therapy significantly. There was not significant effect of adjuvant chemotherapy in stage III in which more fluoropyrimidine and platinum combination was applied. Based on these findings of our study, platinum could be assumed to have a detrimental effect in MSI-H gastric cancer. In most previous studies related with adjuvant therapy in MSI-H gastric cancer, fluoropyrimidine and platinum combination was used. Fluoropyrimidine alone as an adjuvant chemotherapy in MIS-H gastric cancer was not evaluated. Therefore, adjuvant effect of fluoropyrimidine alone in MSI-H gastric cancer should be also evaluated in a further study.
In cases of MSI-H colorectal cancers, adjuvant chemotherapy with 5-fluorouracil alone may even have a detrimental effect on survival [8]. Therefore, recent guidelines do not recommend adjuvant chemotherapy with 5-fluorouracil alone in patients with MSI-H colorectal cancer [10,11]. However, it was reported that adding oxaliplatin could overcome this detrimental effect of 5-fluorouracil on patient survival in MSI-H colorectal cancers and that MSI-H alone did not affect survival in the case of adding oxaliplatin to treat stage III patients [21-24]. Inversely, in our study, adjuvant chemotherapy with fluoropyrimidine alone showed a benefit in terms of survival, which was different from the results obtained in colorectal cancer patients. It was reported that MSI status did not influence the survival of patients treated with 5-fluorouracil and the in vitro antitumor activity of 5-fluorouracil in gastric cancer cells [25]. This difference between gastric cancer and colorectal cancer could be attributed to the biologic differences of MSI-H according to tumor type [26]. In our analysis for mutational profiles of MSI-H gastric and colorectal cancers from The Cancer Genome Atlas database, the mutational profile was different between gastric cancer and colon cancer (S5 Fig.). MSI-H colon cancer showed an increased rate of BRAF mutations compared with MSI-H gastric cancer (55% vs. 22%), but MSI-H gastric cancer exhibited more ARID1A, KMT2D, and RNF43 mutations than MSI-H colon cancer (S5 Fig.).
In conclusion, MSI-H tumor in patients with curatively resected gastric cancer had distinct characteristics with older age, intestinal type, other histology than poorly cohesive, signet ring cell type, lower third location, and absent perineural invasion. MSI-H could be a better prognostic marker in curatively resected gastric cancer. In MSI-H gastric cancer, adjuvant chemotherapy could show a survival benefit, which was in contrast to previous prospective studies and should be investigated in a further prospective trial.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Flowsheet for the selection of microsatellite instability-high (MSI-H) patients in cohort 2. SNUBH, Seoul National University Bundang Hospital.
Mutational profiles for microsatellite instable-high (MSI-H) gastric and colorectal cancers from The Cancer Genome Atlas (TCGA) database. (A, B) Genes with high mutation frequency in MSI-H gastric (n=64) (A) and colorectal (n=28) (B) cancers. Upper graph shows the total number of mutations in each cancer type. Left graph shows the p-values from MutSig analysis. Right matrix demonstrates the mutated genes colored by the types of mutations. Each column stands for an individual cancer sample, and each row denotes a gene. (C) Significantly mutated genes in MSI-H gastric and colorectal cancers compared with each other. Left graph shows the p-values for the enrichment of mutations in each cancer type determined by Fisher’s exact test (green for gastric cancer and yellow for colorectal cancer). Right matrix demonstrates the significantly mutated genes in each cancer type, colored by the types of mutations. Each column stands for an individual cancer sample, and each row denotes a gene, except for the top rows that represent the cancer types.
Notes
Conflicts of interest relevant to this article was not reported.
Acknowledgements
This study was supported by grant no. 11-2012-010 from the SNUBH research fund.