Report of the Korean Association of Lung Cancer Registry (KALC-R), 2014
Article information
Abstract
Purpose
The aim of this study was to investigate epidemiology, clinical characteristics and sex differences of patients with lung cancer using nationwide registry in Korea.
Materials and Methods
The Korean Association for Lung Cancer developed a registry in cooperation with the Korean Central Cancer Registry, and surveyed about 10% of lung cancer cases. For this first survey of cases diagnosed in 2014, cases were selected through a systematic sampling method.
Results
Total 2,621 lung cancer patients were surveyed, and the median patient age was 70 years. During the study period, adenocarcinoma was the most frequent histologic type, the proportion of female patients was 28.4%, and women had a better prognosis (median survival, not reached vs. 13 months; p<0.001) than did men for non-small cell lung cancer. The proportion of never-smokers was 36.4%, and never-smoking was more prevalent in women than in men (87.5 vs. 16.0%, p<0.001). Epidermal growth factor receptor (EGFR) mutations were found in 36.8% of stage IV adenocarcinoma patients, and higher in female compared to male patients (51.2 vs. 26.6%, p<0.001). In addition, patients with EGFR mutation showed better survival (median survival, 18 vs. 8 months; p<0.001) than patients without EGFR mutation in these patients.
Conclusion
This is the first survey to gather unbiased nationwide lung cancer statistics in Korea. More than one-third of lung cancer patients had no smoking history. Female had a high proportion of non-smoker, more adenocarcinoma with EGFR mutation and generally better prognosis than male.
Introduction
Lung cancer is a leading cause of cancer-related deaths worldwide, including in South Korea [1]. Although smoking is one of the most important risk factors for lung cancer, the proportion of never-smokers with lung cancer is generally higher in Asian than in Western populations [2,3]. In addition, mutations in the epidermal growth factor receptor (EGFR) gene, which could be used as a therapeutic target, are more frequent in Asian than in Western lung cancer patients [4].
The first nationwide lung cancer survey in South Korea, conducted by the Korean Academy of Tuberculosis and Respiratory Diseases in 1997 [5], found that squamous cell carcinoma was the most common histologic type. In contrast, the second nationwide lung cancer survey, conducted by the Korean Association for Lung Cancer in 2005 [6], found that adenocarcinoma was the leading histologic type and that lack of symptoms was associated with favorable outcomes.
Generally, female sex is a favorable prognostic factor for lung cancer [7,8]. However, many of these studies were performed in limited groups of patients with specific conditions, such as non-small cell lung cancer (NSCLC) alone [9], or in those treated with certain modalities [10].
To build an unbiased and informative lung cancer registry, the Korean Association for Lung Cancer (KALC) developed a registry (KALC-R) in cooperation with the Korean Central Cancer Registry (KCCR), and surveyed about 10% of lung cancer cases. This study investigated epidemiology, clinical characteristics and sex differences in Korean lung cancer patients using data from the KALC-R of the year 2014.
Materials and Methods
1. Study populations and methods
In 2014, the KCCR registered 24,253 patients newly diagnosed with lung cancer. Among these patients, several patients were excluded with KCCR selection criteria (such as excluding patients who enrolled more than one institution), the total survey population was 21,960 patients. The final survey population comprised 13 regional cancer centers and 39 hospitals from which significant number of registrations were made. Sample size in each hospital (cluster) was determined by selection probability based on the number of registration in each hospital. And patients of each hospital were sorted by date of first diagnosis, sex, age, and Surveillance, Epidemiology, and End Results program (SEER) summary stage. Two thousand six hundred forty patients were selected from each of 52 hospitals using a systematic sampling method [11]. In a process of survey, 19 patients with multiple primary cancer were excluded. In conclusion, 2,621 patients were analyzed in this paper.
A standardized protocol was used to collect data regarding patient age, sex, body mass index (BMI), smoking history, histopathologic tumor type, symptoms, performance status (PS), Eastern Corporative Oncology Group (ECOG) score, clinical stage (according to the seventh edition of the TNM International Staging System), treatment modality, results of molecular tests including EGFR mutation and anaplastic lymphoma kinase (ALK) translocation, and survival status. We defined ever-smokers those with any history of smoking prior to index lung cancer and never-smokers as those with no smoking history. Information such as BMI and PS was obtained in the initial date of visit at the time of diagnosis. Patie-nts were followed up until December 2017.
2. Statistical analysis
Continuous variables are expressed as mean±standard deviation (SD) or median (interquartile range [IQR]), and categorical variables as percentages. Continuous variables were compared using a Mann-Whitney U test, and categorical variables using chi-square or Fisher exact test, as appropriate. Risk factors for mortality were analyzed using Cox proportional hazards models. Variables with a p-value of < 0.20 on univariate analysis were used in the multivariate analysis. Survival was analyzed by the Kaplan-Meier method and compared by log-rank tests. All p-values were two-tailed, with statistical significance set at p < 0.05. All statistical analyses were performed using SPSS ver. 20.0 (IBM Corp., Armonk, NY).
3. Ethical statement
The study protocol was reviewed and approved by the Institutional Review Board at the National Cancer Center (NCC2018-0193), which waived the requirement for informed consent due to the retrospective nature of the study.
Results
1. Patient characteristics
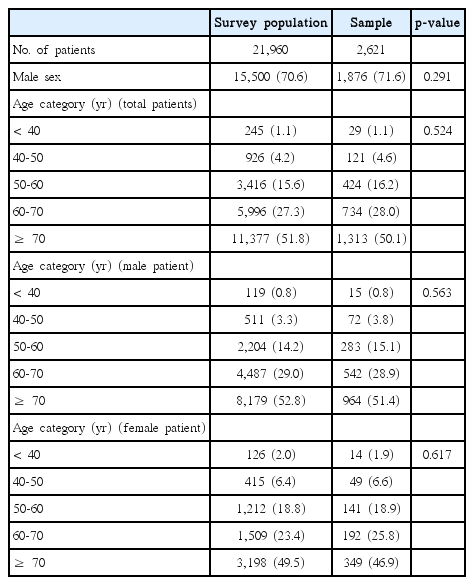
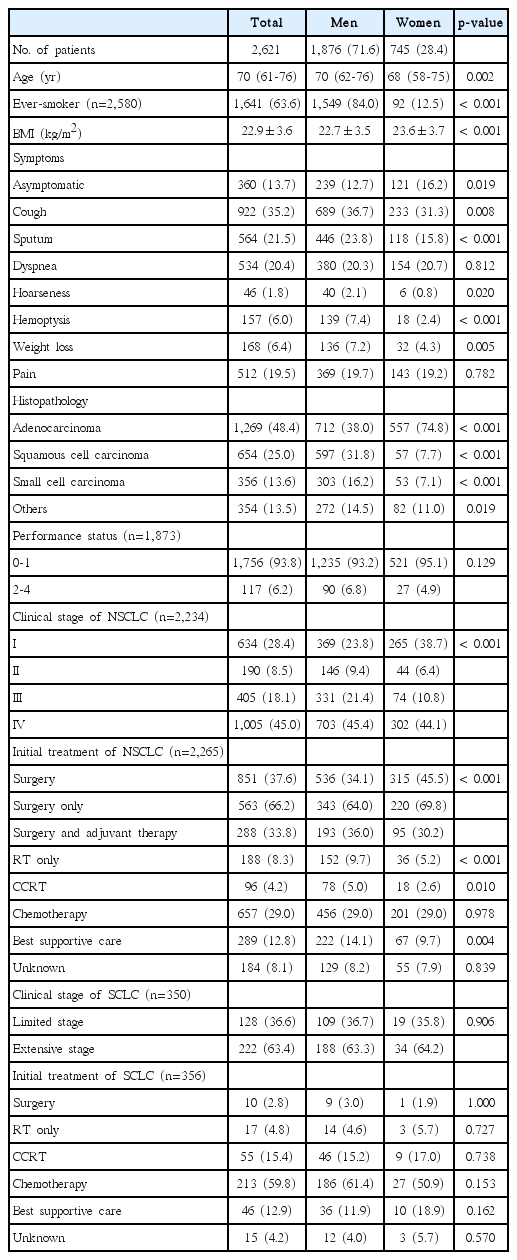
A total of 2,621 lung cancer patients were enrolled at 52 sites in South Korea; there were no differences in sex and age category between survey population and sample (Table 1). Baseline characteristics of enrolled patients are shown in Table 2. The median patient age was 70 years (IQR, 61 to 76 years), 71.6% were men (lower than that in previous studies (79.3% in 1997 and 75.8% in 2005) and men were older than women (median age, 70 years vs. 68 years; p=0.002). Smoking history was significantly more frequent (84.0% vs. 12.5%, p < 0.001) and mean BMI was significantly lower (22.7 kg/m2 vs. 23.6 kg/m2, p < 0.001) in men than in women. Of all patients, 13.7% were asymptomatic at the time of diagnosis, with men being more likely to have symptoms such as cough, sputum, hoarseness, hemoptysis, and weight loss, than women. In case of histopathology, adenocarcinoma (48.4%) was the leading subtype in total patients. While adenocarcinoma was more common in woman (74.8% vs. 38.0%, p < 0.001) compared to male patients, squamous cell carcinoma and small cell carcinoma were more common in male patients.
Among patients with NSCLC, 28.4% had stage I, 8.5% had stage II, 18.1% had stage III, and 45.0% had stage IV disease. The proportion of patients with stage I NSCLC was higher in women (38.7%) than in men (23.8%), whereas the proportion of patients with stage IV tumors was similar (44.1% vs. 45.4%). Among patients with NSCLC, 37.6% of patients received surgery as the initial treatment, 29.0% were treated with chemotherapy, 8.3% received radiation therapy, and 12.8% received no specific anti-cancer treatment. Surgery was significantly more frequent in women than in men (45.5% vs. 34.1%, p < 0.001), whereas radiation therapy (5.2% vs. 9.7%, p < 0.001), concurrent chemoradiation therapy (2.6% vs. 5.0%, p=0.010), and best supportive care (9.7% vs. 14.1%, p=0.004) were significantly less frequent in women.
Among patients with small cell lung cancer (SCLC), 36.6% had limited-stage and 63.4% had extensive-stage disease. Almost 60% of SCLC patients were treated with chemotherapy as the initial treatment, 15.4% received concurrent chemoradiation therapy, and 12.9% received no specific anticancer treatment. There were no sex-associated differences in clinical staging and initial treatment modalities in patients with SCLC.
2. Patient characteristics by smoking history
Among 2,580 patients with smoking history, 939 (36.4%) were never-smokers. The comparison of characteristics between ever-smokers and never-smokers is shown in Table 3. The never-smoker group had fewer men (31.5% vs. 94.4%, p < 0.001), and showed higher BMI (23.4 kg/m2 vs. 22.6 kg/m2, p < 0.001) compared with the ever-smoker group. In addition, the never-smoker group included more asymptomatic patients (16.7% vs. 12.2%, p=0.001), and had better PS than did the ever-smoker group (p=0.045). Among patients with NSCLC, the incidence of stage I disease was higher in never-smokers (36.8%) than in ever-smokers (23.3%), whereas that of stage IV disease was similar (45.5% vs. 44.2%). Among patients with SCLC, never-smokers had more extensive-stage disease than did ever-smokers (75.9% vs. 60.8%, p=0.030).
Clinicopathologic characteristics of patients classified by sex and smoking habits are shown in Table 4. Of the 2,580 patients with available smoking history, 43.3% smoked over 30 pack-years, 20.3% smoked less than 30 pack-years, and 36.4% were never-smokers. The proportions of women in these three groups were 2.8%, 11.6%, and 68.5%, respectively. There were no sex-associated differences in clinicopathologic characteristics of patients who ever-smoked. However, assessments of never-smokers showed that women were younger (median, 68 years vs. 71 years; p=0.003), were more likely to have adenocarcinoma histology (79.9% vs. 56.1%), and had an earlier NSCLC clinical stage than did men.
3. Prognostic factor and survival analysis
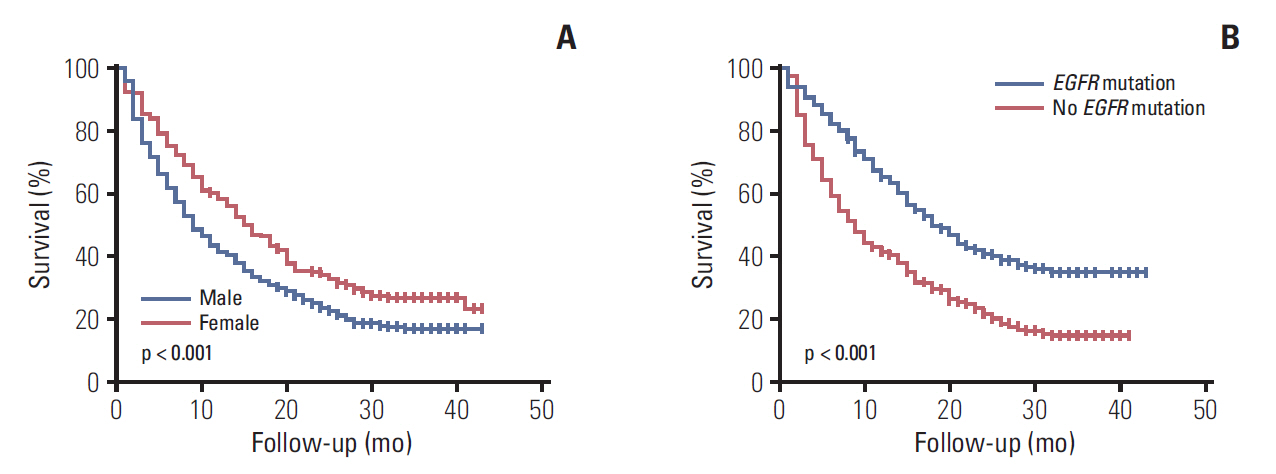
The 2,265 NSCLC patients were followed up for a median of 17 months (IQR, 5 to 32 months), during which 1,321 patients (58.3%) died. Univariate Cox analysis showed that old age, male sex, ever-smoker status, low BMI, poor PS, higher clinical stage, squamous cell carcinoma histology (compared with adenocarcinoma), and non-surgical therapy or best supportive care (compared with surgery) were significant predictors of mortality. On multivariate Cox analysis, these clinical variables, except for ever-smoker and squamous cell carcinoma histology, remained significant prognostic factors (Table 5). Among NSCLC patients, median survival was significantly longer for women than for men (not reached vs. 13 months, p < 0.001) (Fig. 1A) and survival significantly differed with clinical stage at diagnosis (Fig. 1B).
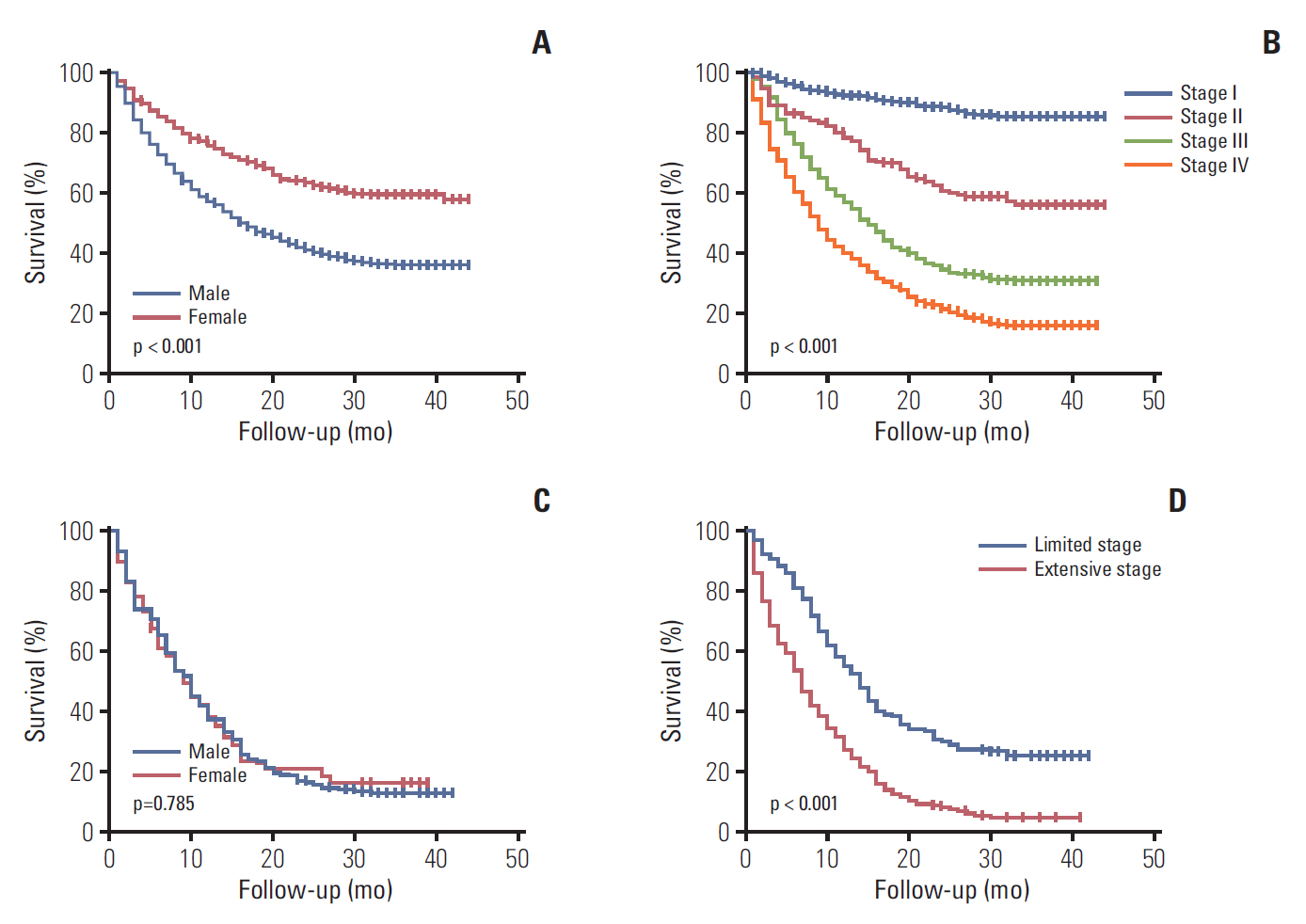
Overall survival in patients with non-small cell lung cancer, according to sex (A) and clinical stage (B). Overall survival in patients with small cell lung cancer, according to sex (C) and clinical stage (D).
The 356 SCLC patients were followed up for a median of 8 months (IQR, 2 to 16 months), during which 306 patients (86.0%) died. Univariate and multivariate Cox analyses showed that older age, lower BMI, poor PS, extensive stage, and best supportive care were significant predictors of mortality (Table 6). Although ever-smoker seemed to be a favorable prognostic factor on univariate analysis, ever-smokers were younger and tended to have a more limited stage than did never-smokers (S1 Table). Although median survival was similar in men and women with SCLC (8 months vs. 8 months, p=0.785) (Fig. 1C), survival significantly differed with clinical stage at diagnosis (Fig. 1D).
4. Molecular profile and survival analysis among patients with adenocarcinoma in the year 2014
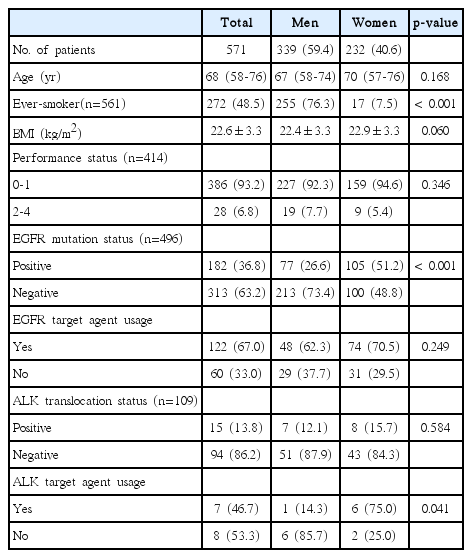
Of the 571 patients diagnosed with stage IV adenocarcinoma, 339 (59.4%) were men (Table 7). EGFR mutations were analyzed in 496 (86.9%) patients, and 182 patients (36.8%) had mutations. Although mutation frequency was significantly higher in women than in men (51.2% vs. 26.6%, p < 0.001), the proportion of mutation-positive men and women treated with a targeted agent did not differ. ALK translocation was analyzed in 109 patients and 15 patients (13.8%) showed ALK translocation. Although translocation frequency was not different among women and men (15.7% vs. 12.1%, p=0.584), the proportion treated with a targeted agent was higher in women than in men (75.0% vs. 14.3%, p=0.041).
Patients with stage IV adenocarcinoma were followed up for a median of 10 months (IQR, 3 to 23 months), during which 437 patients (76.5%) died. On univariate Cox analysis, older age, male sex, ever-smoker status, lower BMI, and poor PS were associated with mortality, and target driver mutation (EGFR mutation and/or ALK translocation) was associated with a favorable outcome. On multivariate Cox analysis, old age, low BMI, poor PS, and target driver mutation remained significant independent predictors of mortality (S2 Table). Women had improved survival (median 15 vs. 8 months, p < 0.001) (Fig. 2A) compared to men in patients with stage IV adenocarcinoma. In addition, patients who harbored EGFR mutations showed better prognosis (18 months vs. 8 months, p < 0.001) (Fig. 2B) than did patients without EGFR mutations. Among patients with stage IV adenocarcinoma and EGFR mutations, patients treated with a target agent showed better prognosis (21 months vs. 13 months, p=0.027) than those who were not treated.
Discussion
Relative to patients of other ethnicities, Asians with lung cancer have generally been reported to have a lower prevalence of smoking and a higher prevalence of tumor-associated EGFR mutations; these factors may be responsible for the more favorable prognosis in Asians [12]. Our nationwide survey, showing the clinical characteristics of lung cancer in South Korea, yielded results comparable to those in other studies of Asian patients. In contrast to prior nationwide surveys in Korea, which was conducted by academic societies, this is the first unbiased statistic data of Korean nationwide lung cancer survey.
Unbiased and reliable epidemiological data are important to identify time trends in diseases and to establish government policy. Although two previous nationwide surveys were performed in South Korea, potential selection bias could not be ruled out during registration of cases at the individual hospital level. Since all patients with confirmed disease codes of C34 are registered in the KCCR, KCCR data have the advantage of including nearly all lung cancer patients without selection bias. The KALC and KCCR have formulated a lung cancer-specific registration system (KALC-R), which has about 80 data fields for demographic data, symptoms, smoking history, histological findings, staging data, molecular study results, and initial treatment details [13]. Recently, our group reported on a pilot survey of 489 patients diagnosed with lung cancer in 2013 [14]; the demographic characteristics were comparable with those from other studies based on the nationwide registry [15,16]. In the current study, among 24,253 newly diagnosed lung cancer patients, about 10% were surveyed from 52 hospitals by a systematic sampling method, which helped eliminate potential bias in sample selection.
The epidemiology of lung cancer in South Korea have changed over time, major recent changes in epidemiology are summarized in S3 Fig. Compared to previous registry-based studies, the incidence of adenocarcinoma increased over the study period in both men and women. In 1997, although the most frequent histological type was squamous cell carcinoma (44.7%), adenocarcinoma incidence has increased over time, and has been the most incident histologic type since 2005. This observation may be explained by changes in cigarette components as well as by risk factors not related to smoking such as occupational risk and air pollution [17]. In addition to changes in histologic type prevalence, the development of screening methods such as low-dose chest computed tomography have led to an increased incidence of asymptomatic, early, and peripherally located cancers [18]. Thus, percutaneous lung biopsy has been most commonly performed for tissue confirmation. Additionally, the frequent use of positron emission tomography with computed tomography (PET-CT) has resulted in increased proportions of patients with stage IV lung cancer. Moreover, the use of PET-CT has made evaluation and characterization of the mediastinal lymph node more feasible; the endobronchial ultrasound-guided transbronchial needle aspiration has gained popularity and is an important diagnostic tool, especially in clinically evident N2 NSCLC [19].
Although smoking is one of the most important causes of lung cancer, the prevalence of cigarette smoking is decreasing in Korea, according to the Korea National Health and Nutrition Examination Survey [20]. In addition, the magnitude of the effect of smoking on lung cancer is smaller in Asian than in Western populations, a phenomenon termed the smoking paradox [21]. Asians are generally older at smoking initiation than are Westerners, and prefer less toxic or filtered tobacco. In contrast, the incidence of lung cancer among never-smokers is higher in Asian populations [2]. Genetic differences such as higher EGFR gene mutation rates in Asian populations and poor ventilation combined with the use of coal may explain these findings [22]. Indeed, about 87.5% of women patients in this survey had no smoking history. Additional studies are required to determine the potential risk factors for lung cancer in never-smokers.
Compared with previous registry-based studies, the incidence of lung cancer in women was higher in this study. Among patients with NSCLC, women had more favorable outcomes than did men, in agreement with previous studies showing better survival outcomes in women than in men with lung cancer [9,23]. Generally, women are diagnosed with lung cancer at a younger age and include a higher percentage of non-smokers, and both these factors are associated with more favorable outcomes. These differences may partly be due to differences in genetic background and hormonal status [24], which affect susceptibility to carcinogens. In addition, because women are more likely to have adenocarcinoma primarily located peripherally [25], the impact of early diagnosis on survival may be greater in women than in men. EGFR mutations are more frequent in women than in men [26], which may contribute to survival advantages. Treatment with targeted agents has been associated with favorable outcomes in patients positive for EGFR mutations and may contribute to the sex differences in survival [27]. However, sex was not associated with survival in patients with SCLC, in contrast to findings from studies in Western populations [28,29]. Among the 356 SCLC patients, only 53 (14.9%) were women. Although sex was not a prognostic factor with statistical significance in this small group of SCLC patients, additional studies in larger populations are needed to confirm this finding.
Among patients with stage IV adenocarcinoma and tested for EGFR mutation, 36.8% were positive for EGFR mutations. EGFR mutations were more common in women than in men, similar to the findings of previous studies in Asian populations [4]. Generally, compared to Western populations, Asians show higher EGFR mutation rates; this may contribute to the more favorable clinical outcomes observed in Asians [30]. The present study found that target driver mutations including EGFR mutations were significant prognostic factors, even after adjusting for other clinical variables. Although EGFR tyrosine kinase inhibitors have shown efficacy [27], EGFR mutation tests were not performed in all of our patients, though it is possible that test data were not provided during this survey for some cases. However, physicians should be aware of the need to test for EGFR mutations in all patients with advanced adenocarcinoma.
This study had several limitations. First, the retrospective study design resulted in an unavoidable deficiency of information such as second-hand smoking status and risk factors other than smoking. However, the strength of the study lies in the unbiased sampling method used to sample a representative population of patients with lung cancer. Second, disease-free survival and progression-free survival rates, as well as complete treatment modalities, could not be analyzed. However, overall survival rate is an acceptable, clear-cut, and important primary outcome. Finally, although the number of patients in this study was relatively large, it was not adequate to perform meaningful comparisons within subgroups with specific conditions. However, we believe that the present study will help in understanding the current trends in the epidemiology of lung cancer and may serve as a reference for future studies.
In conclusion, the evaluation of this unbiased nationwide survey dataset revealed female had a high proportion of non-smoker, more adenocarcinoma with EGFR mutation and generally better prognosis than male. And more than one-third of lung cancer patients had no smoking history. The results of this study show that the development of more effective early lung cancer screening and health insurance programs for lung cancer are indicated.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Baseline characteristics of small cell lung cancer (SCLC) patients according to smoking history
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This study was supported by the Health Promotion Fund, Ministry of Health & Welfare, Republic of Korea (1660680). The data used for this study were provided by the KALC and the Ministry of Health and Welfare, KCCR.