Subdivision of Nasopharyngeal Carcinoma Patients with Bone-Only Metastasis at Diagnosis for Prediction of Survival and Treatment Guidance
Article information
Abstract
Purpose
The purpose of this study was to subdivide M1 stage nasopharyngeal carcinoma (NPC) patients with bone-only metastases for prognosis prediction while identifying the treatment effect of locoregional radiotherapy (LRRT) and metastasis radiotherapy (MRT) among patients with different risk.
Materials and Methods
From November 2006 to October 2016, a total of 226 patients with bone-only metastasic NPC were retrospectively enrolled. All patients developed distant lesions before receiving treatment. All potential prognostic factors were considered and the correlation of the M1 subdivisions with overall survival (OS) was determined by Cox regression hazards model. Kaplan–Meier curves were used to appraise survival condition and log-rank testing was used to compare the differences.
Results
The median follow-up time was 33.9 months (range, 3 to 126 months). According to multivariate Cox proportional hazard analysis, the number of metastatic lesions and Epstein-Barr virus (EBV) DNA status after palliative chemotherapy (PCT) were independent prognostic factors for OS. Thus, we subdivided patients into three risk groups according to these two factors. Systemic chemotherapy combined with LRRT may benefit patients in low- and intermediate-risk groups but not in the high-risk group. Further aggressive MRT based on systemic chemotherapy showed no survival benefit in any risk group.
Conclusion
The stratification of NPC patients with bone-only metastasis based on EBV DNA after PCT and the number of metastatic lesions provided promising prognostic value and could aid clinicians in person-specific treatment.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy considered to be epidemic in east and Southeast Asia, especially in Southern China. Indeed, it has been notoriously described as Canton Tumor. There were 86,700 new cases of NPC and 50,800 deaths reported worldwide in 2012 [1]. NPC is distinguished from other head and neck carcinomas by geographic distribution, where Epstein–Barr virus infection is a major precipitant and has great tendency to result in distant metastases [2]. Due to the concealed location of the nasopharynx, many patients tend to be at an advanced stage of disease at initial diagnosis. A previous study reported that approximately 15% NPC patients were diagnosed as having metastasis before any treatment [3]. Survival times vary widely in NPC patients with distant metastasis at initial diagnosis [4,5]. However, the TNM stage, which is widely used to aid clinicians in prognosis prediction, has shown no value in staging patients diagnosed as having M1 stage. Thus, stratifying the patients with metastatic NPC is necessary for clinicians to classify patients and guide appropriate treatment [6].
Bone metastasis is the most frequently metastatic site, with an estimated incidence of 54%-80% [7-9]. Thus, there is a need for a prognostic model to predict survival risk in those with metastasis. Shen et al. [5] divided bone metastatic NPC into three risk groups using spine involvement factors and metastatic sites number. Similarly, Chen et al. [10] established a prognostic score using six factors to discriminate patients into low-/high-risk groups. However, both studies involved patients who developed metastasis after initial treatment. Besides, Epstein-Barr virus (EBV) DNA, which is an important biomarker for NPC, was not included in the analysis [11]. Platinum-based combination chemotherapy is recommended for metastatic NPC patients according to the National Comprehensive Cancer Network (NCCN) guidelines [12]. However, there is no standard model which considers whether radiotherapy should be applied to patients with primary head and neck tumors with distant metastases. Studies focusing on the application of ablative treatment to the metastasis site are rare.
Therefore, the purpose of our study was to divide patients with bone-only metastasis into different risk groups. Furthermore, we aimed to investigate whether these patients could benefit from locoregional radiotherapy (LRRT) and metastasis radiotherapy (MRT) in the different risk groups.
Materials and Methods
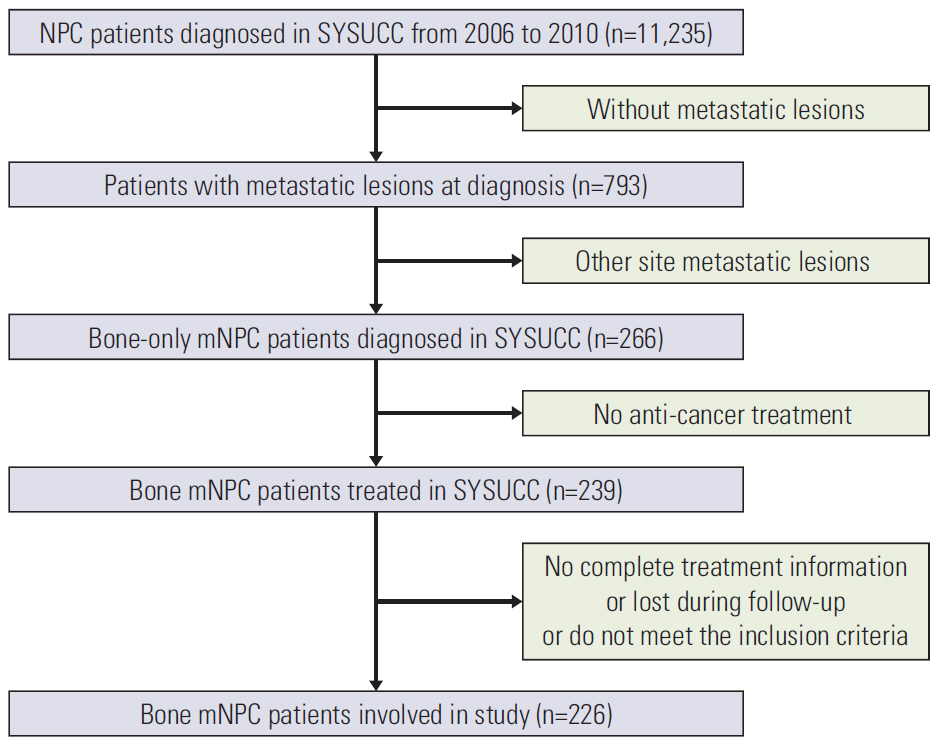
1. Patients
Between November 2006 and October 2016, 226 consecutive newly diagnosed bone-only metastatic NPC patients in Sun Yat-sen University Cancer Center (SYSUCC), China, were enrolled in our analysis. The inclusion criteria were as follows: (1) pathological diagnosis of NPC; (2) cisplatinbased palliative chemotherapy (PCT); (3) Karnofsky performance score > 70; (4) adequate organ function (white blood cell > 4.0×10/L; neutrophil > 2.0×10/L; hemoglobin > 90 g/L; platelet > 100×109/L; aspartate aminotransferase/alanine transaminase < 2.5 upper limit of normal; Ccr > 60 mL/min); and (5) absence of pregnancy, lactation, and other malignant disease. The flow chart is described in Fig. 1. Routine evaluations were performed on patients, including physical examination, electrocardiography, head and neck magnetic resonance imaging (MRI) with contrast, nasopharyngoscopy and biopsy, chest and abdominal computed tomography (CT) with contrast and bone scan. The positron emission tomography computed tomography (PET-CT) was considered as an alternative to whole body examination. Whe-ther patients have initial bone metastasis were based on the image diagnostic report before treatment. Bone metastasis was taken into consideration when either emission computed tomography or PET-CT showed evidence of bone lesions. Besides, other examines or PET-CT could not find metastasis in other organs. These patients were defined as the initial bone-only metastasis patients. Two radiologists determined all image diagnostic reports on the basis of the imaging diagnosis criteria. Plasma EBV DNA measurement was routinely performed and further examinations were considered when necessary.
2. Chemotherapy and radiation therapy
All eligible patients received the cisplatin-based combination PCT. Common PCT regimens included TPF (docetaxel [60 mg/m2 day 1] combined with cisplatin [60 mg/m2 day 1] plus 5-fluorouracil [500-800 mg/m2, 120 hours]), PF (cisplatin [20-25 mg/m2 days 1-3] combined with 5-fluorouracil [800-1,000 mg/m2, 120 hours]), and TP (docetaxel [75 mg/m2, day 1] combined with cisplatin [20-25 mg/m2, days 1-3]). Chemotherapy was administered intravenously every 3 weeks. After PCT, 157 patients and 68 patients experienced LRRT and MRT respectively, using intensity modulated radiation therapy (IMRT) or two-dimensional conventional radiotherapy. The total dose of radiotherapy was 68-70 Gy for the nasopharynx and neck and 30-40 Gy for the metastatic site, 5 times a week, at approximately 2 Gy per fraction. The detailed design of the IMRT plan was generated using previous studies [13].
3. Outcome and follow-up
The primary endpoint of this study was overall survival (OS), which was defined as the time from the date of diagPCT, palliative chemotherapy; EBV, Epstein-Barr virus; TPF, cisplatin plus docetaxel plus 5-fluorouracil; TP, cisplatin plus docetaxel; PF, cisplatin plus 5-fluorouracil; LRRT, locoregional radiotherapy; MRT, metastasis radiotherapy. a) According to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system, b) Undetectable/detectable EBV DNA levels after neoadjuvant chemotherapy are based on a cut-off value of 0 copy/mL.
nosis to the date of death of any cause. Patients were inspected every 3 months in the first 3 years and every 6 months thereafter until death. A series of evaluations were conducted including physical examination, nasopharyngoscopy, MRI with contrast of head and neck, CT/magnetic resonance with contrast of the metastatic sites, abdominal sonography, chest radiography and plasma EBV DNA measurement. PET-CT was considered when necessary.
4. Statistical analyses
Time-to-event data in different subgroups were analyzed using Kaplan-Meier curves and compared with the log-rank test. Univariable and multivariable Cox regression analyses were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between the characteristic and overall survival. Statistical analyses were performed using the SPSS (Mac ver. 21.0, IBM Corp., Armonk, NY). All statistical tests in our study were 2-tailed. p < 0.05 was considered to represent statistical significance.
5. Ethical statement
At our center, all patients signed informed consent prior to treatment, including their consent to treatment and clinical information for further prognostic analysis. This study was approved by the Research Ethics Committee of the Sun Yatsen University Cancer Center, China.
Results
1. Patient characteristics and OS
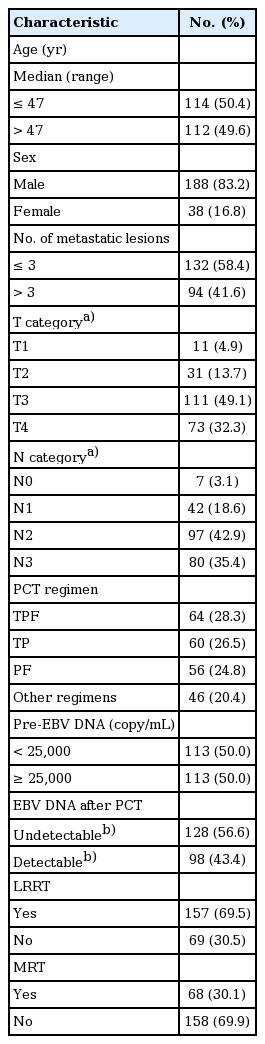
Among all 226 patients, the median age was 47 years (range, 18 to 74 years). Patients in our cohort were from NPC endemic areas with a male predominance (83.2%). All patients underwent first-line PCT. After PCT, 157 patients (69.5%) underwent LRRT and 69 patients (30.1%) underwent MRT. With respect to number of lesions, 94 patients (41.6%) had more than three metastatic lesions. EBV DNA was detected in 207 patients (91.6%) before PCT and only 98 patients (43.4%) had detectable EBV DNA levels after PCT. Other characteristics of the 226 patients are listed in Table 1. The median follow-up time was 33.9 months (range, 3 to 126 months). During follow-up, 105 patients died of tumor progression and two patients died of treatment-related toxicities.
2. Univariable analysis and multivariable analysis
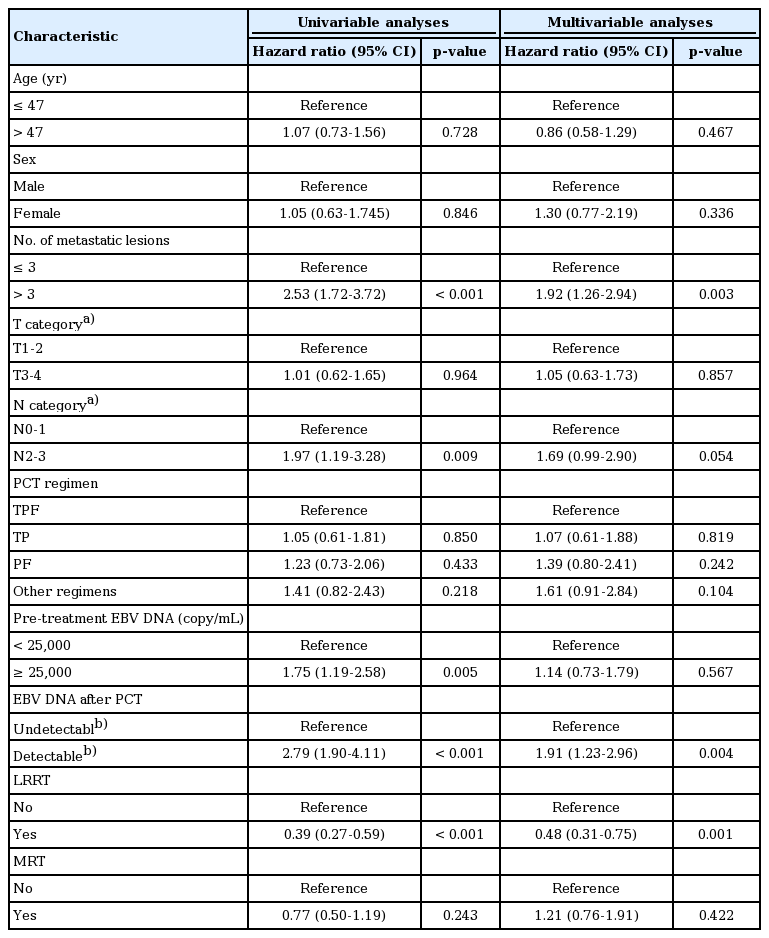
All potential prognostic factors were considered in the Cox proportional hazards model. The cut-off value of the number of metastatic lesions for patient outcome was determined by receiver operating characteristic (ROC) curve analysis (3 metastasis lesions). The pre-treatment EBV DNA level was categorized based on the median value (25,000 copies/mL). The EBV DNA after PCT was scored on the basis of a detectable/undetectable scale (a cut-off value of 0 copy/mL). Univariate analysis showed that the number of metastatic lesions, N category, pre-treatment EBV DNA, EBV DNA post-PCT, and LRRT were significantly associated with OS among patients with bone metastasis at primary diagnosis. In contrast, only the number of lesions, EBV DNA post-PCT, and LRRT remained significant in the multivariate analysis (Table 2). The Kaplan–Meier survival curves in patients are shown in Fig. 2A and B.
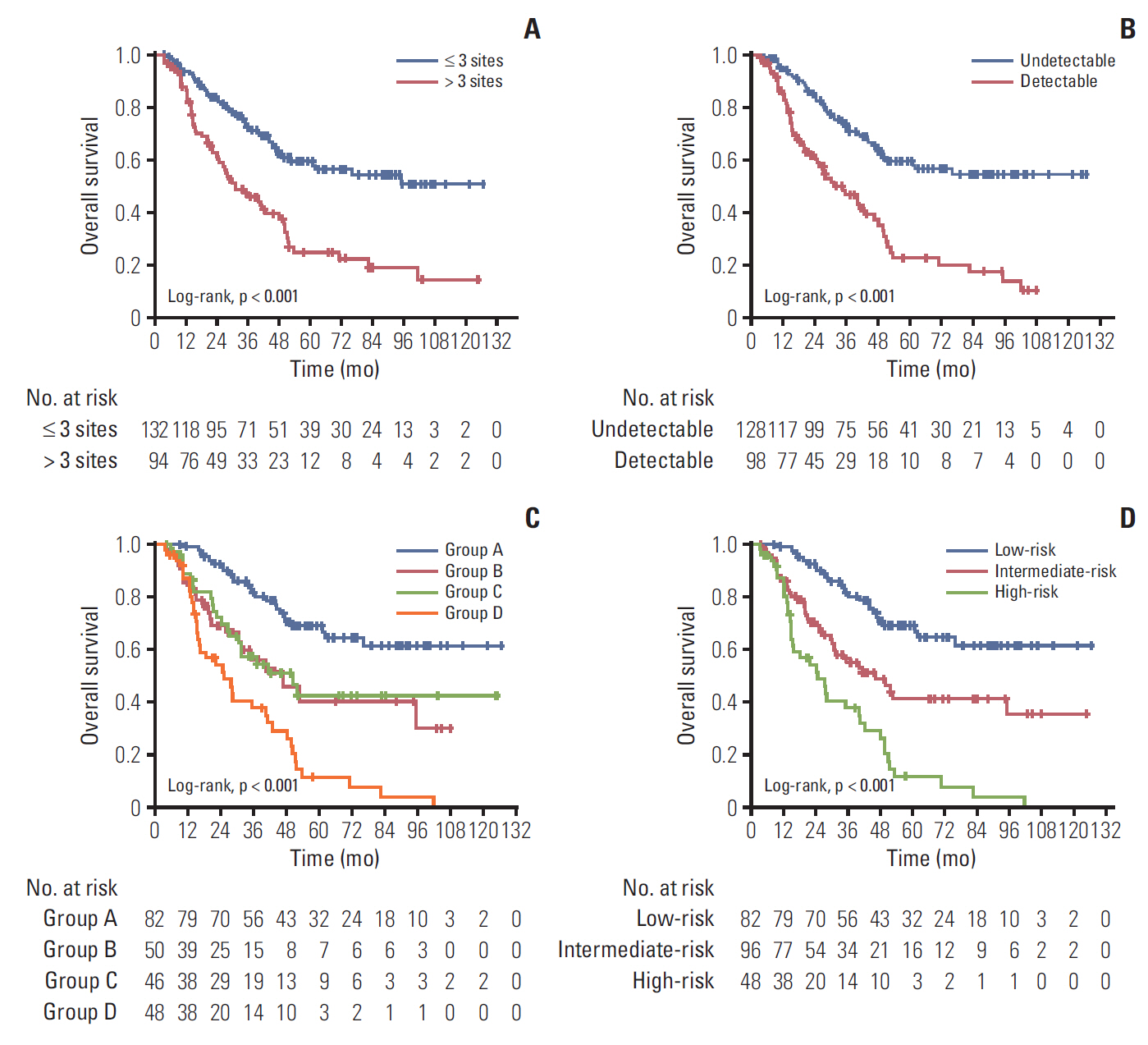
(A) Kaplan-Meier overall survival curves in 226 patients with bone-only metastatic nasopharyngeal carcinoma. Patients grouped by the number of metastatic lesions by Epstein-Barr virus (EBV) DNA after palliative chemotherapy (PCT) (B); by combination of lesion numbers and EBV DNA after PCT (C) and by risk stratification (D).
3. Risk stratification
According to the independent prognostic factors, we subdivided all patients into four subgroups: group A, three or fewer metastatic lesions and undetectable EBV DNA after PCT; group B, more than three metastatic lesions and undetectable EBV DNA after PCT; group C, three or fewer metastatic lesions and detectable EBV DNA after PCT; and group D, more than three metastatic lesions and detectable EBV DNA after PCT. Further pair-wise comparisons showed that group B and group C were not significantly different (p=0.764), and that they both had significantly lower survival rates than group A. They also had a higher survival rate than group D (S1 Table). Thus, we combined group B and group C as the intermediate-risk subgroup, with groups A and D serving as the low-risk and high-risk groups, respectively. Kaplan-Meier survival curves are shown in Fig. 2C and D. The survival rate and multivariate analysis for patients in different risk groups are listed in Table 3.
4. Treatment outcome for those in different risk levels
We further explored the impact of combined LRRT or MRT among patients with bone-only metastatic NPC in different risk groups. In the low- and intermediate-risk groups, the OS rate was significantly higher in patients treated with LRRT (p=0.006 and p=0.005, respectively), while there was no survival benefit observed in high-risk patients (p=0.918) (Fig. 3A-C). Furthermore, patients in any risk group received no benefit from MRT (Fig. 3D-F). Then, we conducted stratified multivariate analysis for the different risk groups. HR was adjusted for age, sex, and other confounding variables. Fig. 4 showed the six groups of patients stratified by treatment modality and risk groups. Adjusted HR for OS increased from those in low-, intermediate-, and high-risk groups in all treatment modalities. LRRT was an independent prognostic factorin the low- and intermediate-risk groups (HRlow, 0.23; 95% CI, 0.09 to 0.61; p=0.003; HRintermediate, 0.40; 95% CI, 0.21 to 0.76; p=0.005). However, MRT was not associated with a better OS in all groups of patients (Table 4).

Comparison of overall survival of patients in the locoregional radiotherapy (LRRT) and non-LRRT group: low-risk patients (A), intermediate-risk patients (B), and high-risk patients (C). Comparison of overall survival in patients in the metastasis radiotherapy (MRT) and non-MRT group: low-risk patients (D), intermediate-risk patients (E), and high-risk patients (F).
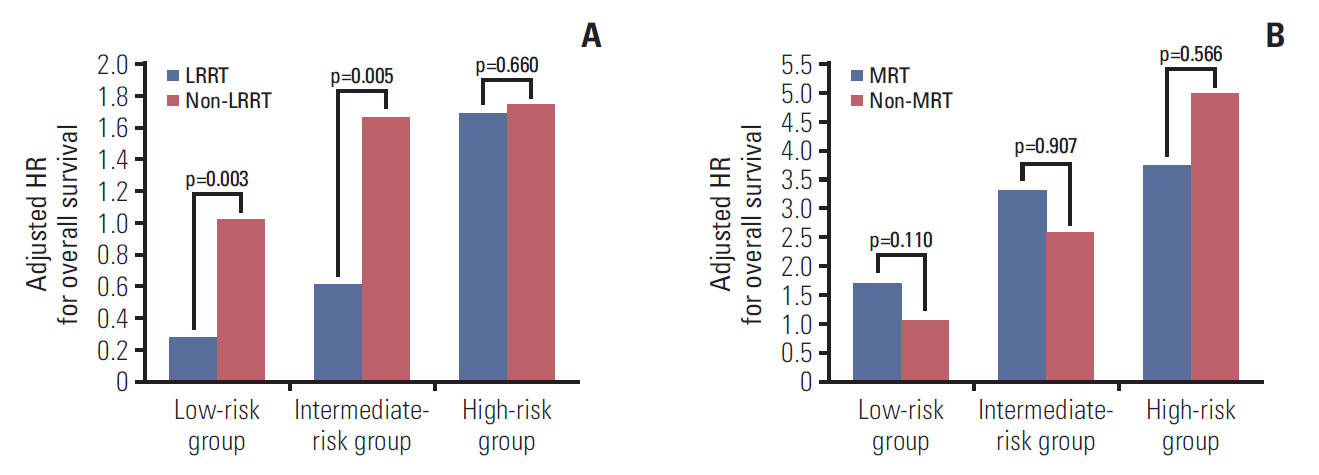
Adjusted hazard ratios (HRs) for overall survival stratified by risk group and locoregional radiotherapy (LRRT) (A) or metastasis radiotherapy (MRT) (B) in patients with bone-only metastatic nasopharyngeal carcinoma. The low-risk group not combined with LRRT/MRT was the reference group (HR, 1). The adjusted variables were age, sex, T category, N category, and pre-treatment Epstein-Barr virus DNA.
Discussion
Bone is the most frequently involved organ in metastatic NPC patients [7-9]. In this study, we included 226 NPC patients with bone metastasis at primary diagnosis. This was based on the large cohort of bone-only metastasis NPC patients from endemic areas. OS among these patients ranged from 3 to 126 months, which indicates that long-term survival is possible in these patients. Therefore, it is necessary to find reliable prognostic factors that can be used to predict survival more accurately, and guide appropriate treatment. Consequently, we explored the prognostic factors and the effect of treatment methods among them. There were several notable finding in our study. First, the number of metastatic lesions and EBV-DNA level are independent prognostic factors for OS in NPC patients with bone metastasis and these could be used as a basis for risk stratification. Secondly, LRRT was a valid therapeutic method, which should be combined with PCT in low- and intermediate-risk patients. Thirdly, MRT did not confer any survival benefit in any risk groups in our analysis. Thus, local treatment of distant metastasis should be carefully considered in clinical application.
EBV DNA, which is measured by real-time quantitative polymerase chain reaction, has been demonstrated to be closely associated with NPC [14]. In non-metastatic NPC patients, several studies have verified the prognostic value of EBV DNA levels before treatment (pre-EBV DNA) and EBV DNA levels after concurrent chemoradiation therapy/radiotherapy (post-EBV DNA) [15,16]. In local advanced patients NPC who undergo induction chemotherapy (IC) before radiotherapy, our previously study showed that patients had undetectable EBV DNA after IC achieved higher progression-free survival and distant metastasis-free survival compared with patients who had detectable EBV DNA after IC [17]. Moreover, in metastatic/recurrent NPC patients, the predictive value of EBV for prognosis has also been demonstrated [18]. In our study, the plasma EBV DNA level was measured before treatment and after PCT in all patients. Univariate analysis showed that the EBV DNA level at these two time points was both associated with OS of NPC patients with bone-only metastasis, while detection of EBV DNA after PCT remained significant in multivariate analysis, indicating that undetectable EBV DNA after PCT was a better prognostic factor in these types of patients.
The number of metastatic lesions was closely related to the tumor burden. In metastatic NPC patients, several studies have demonstrated that patients with single metastasis achieve higher OS compared with patients with multiple metastases [19,20]. According to the results of studies assessing other types of malignancy, patients with multiple bone metastases also had poor clinical outcome [21-23]. However, few studies have focused on the prognostic value of the lesion number in survival among NPC patients with bone-only metastasis. In our study, the cut-off values of lesion number for OS was three based on the ROC analysis. We then considered this as a potential prognostic factor, which was inclu-ded in univariate and multivariate analysis. It is intriguing that the number of lesions was an independent factor for OS in the multivariate analysis. Our result was in accordance with a previous study [5]. These findings suggest that more than three metastatic lesion could be used to establish risk stratification in bone-only metastatic NPC patients.
The treatment strategy for metastatic NPC patients remains a subject of great debate [24]. Platinum-based PCT is the most widely used treatment in these types of patients with objective response rates of 55%-80% [25,26]. Recently, several studies demonstrated that local control of primary tumor by radiotherapy could prolong survival [4,19,27]. Moreover, according to the NCCN guidelines, LRRT could benefit metastatic NPC patients with limited sites or with low tumor burden [12]. Thus, there still remains the issue of who will benefit from the combined treatment and further studies are required to resolve this. In the stratified analysis, we found that LRRT significantly improved OS in low- and intermediate-risk patients but conferred no benefit to high-risk patients. In accordance with the American College of Radiology therapeutic guidelines for bone metastasis, the management of patients with serious illnesses or conditions should concentrate on improving end-stage quality of life and maintaining neurological function [28]. In effect, aggressive treatment is not a suitable treatment for such individuals.
There is no consensus on whether local treatment of metastatic lesions is associated with a better OS in bone metastasis NPC patients due to the limited number of studies, which comprise small numbers of cases. In some case reports, radiotherapy of metastatic lesions has been observed to bring survival benefits for patients with bone-only oligometastatic lesions [29,30]. However, according to our results, all patients did not attain survival benefit from MRT, even for patients considered to be low risk. It has been suggested that better survival outcome in patients with bone-only oligometastatic lesions may be due to systemic PCT and LRRT, and not to MRT.
There were several limitations to our study. First, ours was a retrospective study so the evidence supporting these conclusions is not sufficient or convincing because of the potential for selective bias. Secondly, the cohort was obtained from an endemic area in one treatment center and may not be generally representative of patients with bone-only metastatic NPC. Finally, only 226 patients were involved in the study duo to the low incidence rate. For these reasons, a multiinstitutional prospective study is necessary to validate our results in the future.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.