Associations of Genetic Variations in Mismatch Repair Genes MSH3 and PMS1 with Acute Adverse Events and Survival in Patients with Rectal Cancer Receiving Postoperative Chemoradiotherapy
Article information
Abstract
Purpose
Mismatch repair (MMR) deficiency plays a critical role in rectal cancer. This study aimed to explore the associations between genetic variations in seven MMR genes and adverse events (AEs) and survival of patients with rectal cancer treated with postoperative chemoradiotherapy (CRT).
Materials and Methods
Fifty single nucleotide polymorphisms in seven MMR (MLH1, MLH3, MSH2, MSH3, MSH6, PMS1 and PMS2) genes were genotyped by Sequenom MassARRAY method in 365 patients with locally advanced rectal cancer receiving postoperative CRT. The associations between genotypes and AEs were measured by odds ratios and 95% confidence intervals (CIs) by unconditional logistic regression model. The associations between genetic variations and survival were computed by the hazard ratios and 95% CIs by Cox proportional regression model.
Results
The most common grade ≥ 2 AEs in those 365 patients, in decreasing order, were diarrhea (44.1%), leukopenia (29.6%), and dermatitis (18.9%). Except 38 cases missing, 61 patients (18.7%) died during the follow-up period. We found MSH3 rs12513549, rs33013 and rs6151627 significantly associated with the risk of grade ≥ 2 diarrhea. PMS1 rs1233255 had an impact on the occurrence of grade ≥2 dermatitis. Meanwhile, PMS1 rs4920657, rs5743030, and rs5743100 were associated with overall survival (OS) time of rectal cancer.
Conclusion
These results suggest that MSH3 and PMS1 polymorphisms may play important roles in AEs prediction and prognosis of rectal cancer patients receiving postoperative CRT, which can be potential genetic biomarkers for rectal cancer personalized treatment.
Introduction
In China, colorectal cancer (CRC) ranks the fifth leading cause of cancer death and approximately 300,000 new CRC cases occur each year [1]. Rectal cancer accounts for approximately 30% of all CRC. Individuals with high-risk stage II or III rectal cancer are commonly treated with adjuvant chemoradiotherapy (CRT) combined with surgery [2]. Patients vary in their response to the postoperative CRT [3]. A lot of them suffer from adverse events (AEs) or tumor progression or even death. As we all known, familial risk is one of the highest among cancers, suggesting that genetic factor may play an important role in rectal cancer. Therefore, inherited genetic factors may explain some of the variability of patients’ response and prognosis [4,5]. If genetic markers of predicting the risk of AEs and prognosis can be reliably identified as we previously found [6], they might offer insights into the biological mechanism of response to treatment and prognosis.
Mismatch repair (MMR) is one of the most important DNA repair processes that play key roles in the correction of mispairs arising during DNA replication and participate in apoptotic signaling in response to DNA damage [7,8]. When a germ-line microsatellite allele has gained or lost repeat unit, MMR ability has been disrupted [9]. MMR deficiency (dMMR) has been associated with prognosis and response to fluoropyrimidine-based adjuvant therapy in CRC [10]. Emerging evidence suggests that dMMR can be predictive of significant response and survival gain from immune checkpoint inhibitors [11,12]. It was reported that genetic variations in MMR genes were associated with survival and chemotherapy toxicity in different types of cancer [13,14]. However, MMR status considered as one of the most well-established biomarkers in CRC, little has known about the associations between MMR polymorphisms and AEs and survival in rectal cancer.
Based on the above considerations, the purpose of this study was to investigate the role of MMR genetic polymorphisms in modulating the risk of AEs and prognosis of rectal cancer. It is essential to understand the involvement of MMR system in the response of cancer cells to anticancer treatment for determining individualized CRT strategy that allows selecting patients most likely to benefit from limit toxic effects and maximize efficacy.
Materials and Methods
1. Patients
All of the 365 Chinese patients with rectal cancer recruited at the Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) between January 2005 and June 2015 were treated with total mesorectal excision surgery followed by a total radiation dose of 50 Gy applied in 25 fractions over a period of 5 weeks concurrently with daily administration of CAP (1,600 mg/m2 per day, continuously for 2 weeks and taking a week off every 21-day cycle [days 1 through 14 and days 21 through 35]). Study details were described in full in our prior publication [6]. Patient eligibility criteria included as following: (1) diagnosed by at least two certified pathologists as rectal adenocarcinoma; (2) primary, locally advanced rectal cancer without distant metastases; (3) Karnofsky Performance Score (KPS) ≥ 70 and life expectancy ≥ 6 months; (4) not more than 75 years old; and (5) with no history of cancer and with adequate organ function. AEs were graded according to the Common Terminology Criteria for Adverse Events ver. 4.0.
A total of 365 unrelated patients were eligible to participate in this analysis. Demographic and clinical characteristics information (including sex, age, clinical stage, tumor grade, KPS, surgical procedure, and tumor location) were obtained from each patient’s medical records. Patients were followed up to get their survival information and follow-up data were obtained at outpatient clinic and medical records or by telephone call. The last follow-up date was June 22, 2018.
2. Single nucleotide polymorphisms selection and genotyping
There are a great number of single nucleotide polymorphisms (SNPs) located in these seven MMR genes. We applied candidate strategy to select SNPs of interest. First of all, we searched all SNPs in the National Center for Biotechnology Information dbSNP databases in these seven MMR genes. Second, we sifted out SNPs with minor allele frequency ≥ 0.10 according to the Chinese Han Beijing population data of 1000 GENOMES. Then, we choose SNPs according to haplotype blocks of the genetic variations among Han Chinese with r2 > 0.8 using HaploReg. Finally, 50 candidate SNPs in those seven MMR genes were selected for study (S1 Table).
Whole blood sample (2 mL) was collected from patient at the time of enrollment before therapy, and DNA was extracted using the manufacturer’s recommendations. All polymorphisms in MMR genes were genotyped by Sequenom MassARRAY method. Primers and probes used were shown in S2 Table. For quality controls, genotyping was performed with one blank sample and four replication samples included in each 96-well plate without knowledge of patients’ information. The reproducibility was 100%.
3. Statistical analysis
AEs were grouped into grade 2 to 4 leukopenia, grade 2 to 4 diarrhea, and grade 2 or 3 dermatitis. The associations between genotypes and AEs were measured by odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for sex, age, clinical stage, tumor grade, KPS, surgical procedure, and tumor location by unconditional logistic regression model. Overall survival (OS) was calculated as the time to death or last follow-up from the date of diagnosis. Univariate Cox hazard proportional models were used to evaluate the effect of genotypes on the OS without adjusting for any clinical characteristics while multivariate analysis was adjusted for sex, age, clinical stage, tumor grade, KPS, surgical procedure, and tumor location. Survival distributions were estimated using the Kaplan-Meier method and compared with log-rank test. The statistical significance was settled at p < 0.05. All the statistical tests were performed by using software R v3.3.3 and SPSS ver. 21.0 (IBM Corp., Armonk, NY).
4. Ethical statement
Written informed consent for genotypic analysis was specifically obtained from each patient and this study was approved by the Institutional Review Board of Cancer Hospital, Chinese Academy of Medical Sciences and met the requirements of Declaration of Helsinki.
Results
1. Patient characteristics and clinical outcomes
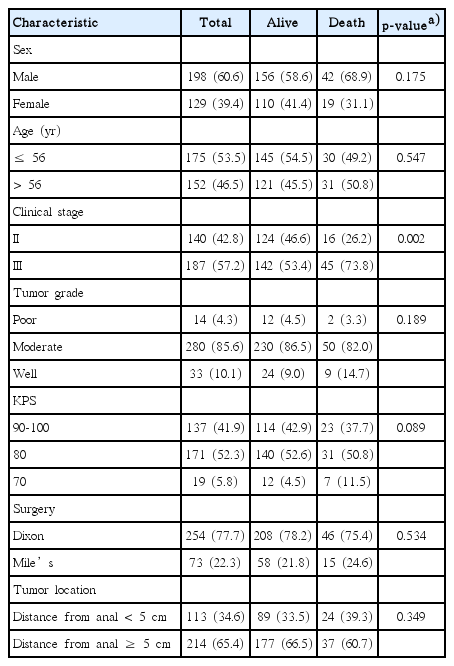
Patients were grouped according to sex, age, clinical stage, tumor grade, KPS, surgery, and tumor location. Baseline clinical characteristics of 365 patients with rectal cancer were shown in Table 1. Grade ≥ 2 leukopenia occurred less frequently in male than in female (p=0.028). Surgical treatments and tumor location had great impact on the occurrence of grade ≥ 2 diarrhea (p < 0.001 and p < 0.001, respectively) and dermatitis (p=0.002 and p < 0.001, respectively). Baseline clinical characteristics of 327 patients with complete survival data were summarized in Table 2. By the time of final analysis (June 22, 2018), the median follow-up period was 92 months (range, 41 to 161 months), and 61 patients died during the follow-up period. We examined whether the baseline clinical characteristics contribute to survival and found that stage II patients had significantly longer OS than stage III patients (p=0.002). No significant difference in OS was presented between patients with other demographic clinical characteristics.
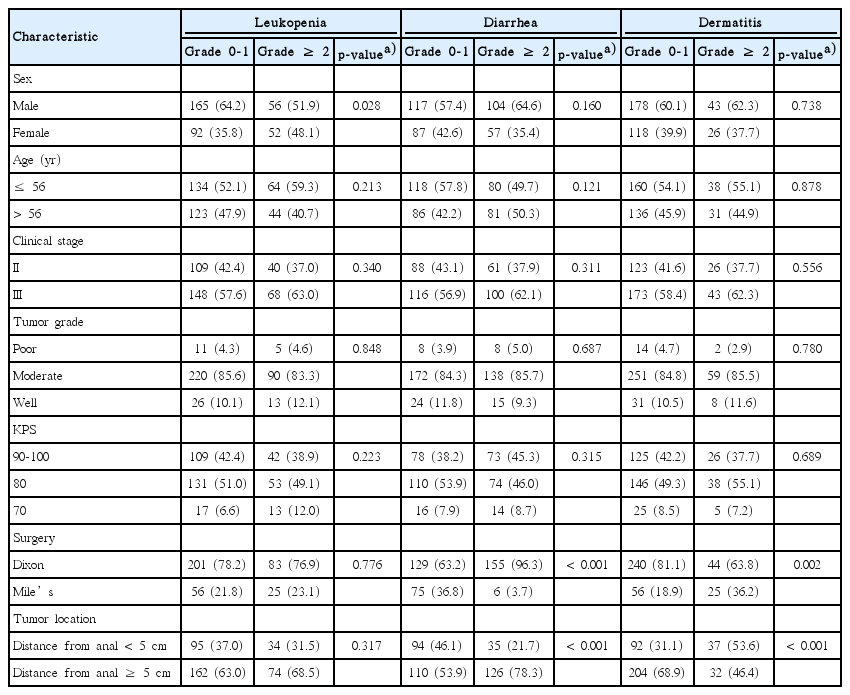
Associations between clinical characteristics and acute adverse events in patients with rectal cancer receiving postoperative chemoradiotherapy
2. Associations between genetic variations in MMR genes and AEs in rectal cancer
We explored whether the genetic variations in MMR genes had an impact on the occurrence of AEs in patients with rectal cancer. The association results were shown in S3 Table. For the 50 candidate SNPs examined in the current study, none of them was significantly associated with the occurrence of grade ≥ 2 leukopenia after adjustment for sex, age, clinical stage, tumor grade, KPS, surgical procedure and tumor location.
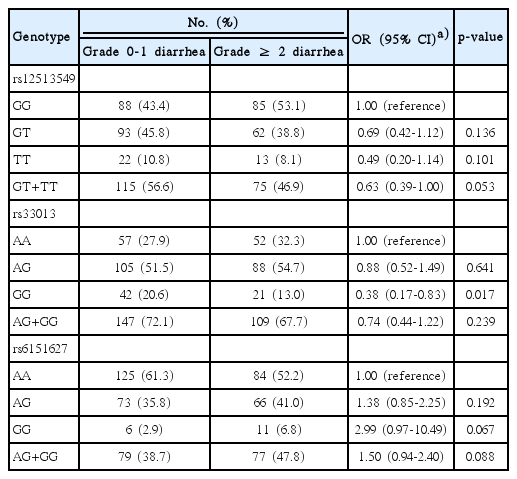
As for the risk of grade ≥ 2 diarrhea, we found 3 SNPs, including rs12513549 (additive model: OR, 0.68; 95% CI, 0.48 to 0.97; p=0.037), rs33013 (OR, 0.71; 95% CI, 0.50 to 0.99; p=0.049), and rs6151627 (OR, 1.51; 95% CI, 1.02 to 2.27; p=0.041), located in MSH3, were significantly associated with diarrhea after adjusting for clinical characteristics (S3 Table). Compared with the rs12513549 GG genotype, the adjusted OR for patients with the rs12513549 GT genotype and TT genotype was 0.69 (95% CI, 0.42 to 1.12; p=0.136) and 0.49 (95% CI, 0.20 to 1.14; p=0.101), respectively. Compared with the rs33013 AA genotype, the adjusted OR for patients with the rs33013 AG genotype and GG genotype was 0.88 (95% CI, 0.52 to 1.49; p=0.641) and 0.38 (95% CI, 0.17 to 0.83; p=0.017), respectively. Compared with the rs6151627 AA genotype, the adjusted OR for patients with the rs6151627 AG genotype and GG genotype was 1.38 (95% CI, 0.85 to 2.25; p=0.192) and 2.99 (95% CI, 0.97 to 10.49; p=0.067), respectively (Table 3). The remaining 47 SNPs were not significantly associated with the risk of diarrhea.
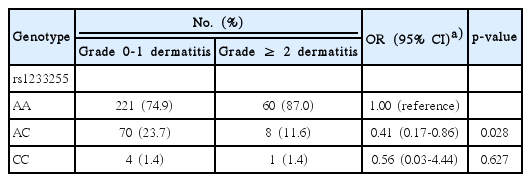
In addition, we found rs1233255 A>C in PMS1 was the only significant SNP associated with the occurrence of grade ≥ 2 dermatitis, with an adjusted OR of 0.48 (95% CI, 0.23 to 0.93; p=0.041) (S3 Table). Compared with the rs1233255 AA genotype, individuals with AC genotype (OR, 0.41; 95% CI, 0.17 to 0.86; p=0.028) or AC genotype combined CC genotype (OR, 0.42; 95% CI, 0.19 to 0.87; p=0.027) had reduced risk of grade ≥ 2 dermatitis, although CC genotype did not reach significance likely because of the small number of individuals carrying CC genotype (OR, 0.56; 95% CI, 0.03 to 4.44; p=0.627) (Table 4).
3. Associations between genetic variations in MMR genes and survival in rectal cancer
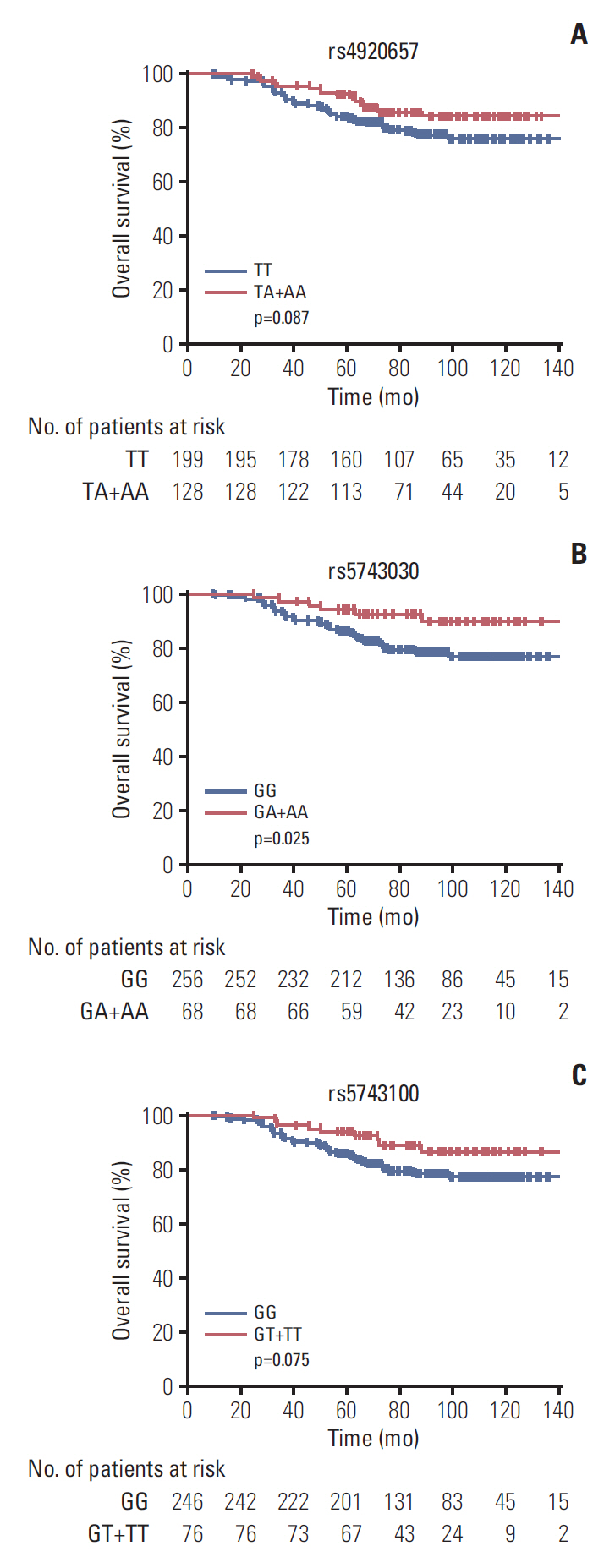
To examine whether genetic polymorphisms associated with rectal cancer survival time, we performed univariate and multivariate Cox regression analysis. The rs4920657, rs5743030 and rs5743100 polymorphisms in PMS1 gene were significantly associated with OS (additive model: hazard ratio [HR], 0.58; 95% CI, 0.36 to 0.94; p=0.026 for rs4920657; HR, 0.36; 95% CI, 0.16 to 0.84; p=0.018 for rs5743030; and HR, 0.49; 95% CI, 0.24 to 0.98; p=0.043 for rs5743100, respectively) (S4 Table). The results of univariate analysis showed differential OS time between genotypes of these three SNPs (dominant model: HR, 0.62; 95% CI, 0.36 to 1.08; p=0.090 for rs4920657; HR, 0.40; 95% CI, 0.17 to 0.92; p=0.031 for rs57430-30; and HR, 0.53; 95% CI, 0.26 to 1.08; p=0.080 for rs5743100, respectively), although the associations of rs4920657 and rs5743100 between genotypes and OS time just reached a borderline-significance. Just like the univariate analysis, the multivariate analysis draw the same conclusion (HR, 0.54; 95% CI, 0.31 to 0.94; p=0.029 for rs4920657; HR, 0.36; 95% CI, 0.15 to 0.85; p=0.019 for rs5743030; and HR, 0.50; 95% CI, 0.25 to 1.03; p=0.060 for rs5743100, respectively) (Table 5). Individuals with the heterozygous and variant homozygous genotypes had longer OS time (log-rank test: p=0.087 for rs4920657, p=0.025 for rs5743030, and p=0.075 for rs5743100, respectively) (Fig. 1), even though Kaplan-Meier analysis of rs4920657 and rs5743100 showed borderline-significant longer OS time.

Associations between 3 SNPs in PMS1 and overall survival time in patients with rectal cancer receiving postoperative CRT
Discussion
In this study, we investigated the associations of 50 SNPs in seven genes involved in MMR pathway (MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2) with AEs and OS in patients with locally advanced rectal cancer. Here we found three SNPs in MSH3, including rs12513549, rs33013, and rs6151627, were associated with the occurrence of grade ≥ 2 diarrhea, and rs1233255 in PMS1 decrease the risk of grade ≥ 2 dermatitis. In particular, three SNPs located in PMS1 (including rs4920657, rs5743030, and rs5743100) could affect OS time of rectal cancer.
MSH3, located in chromosome 5q14.1, the protein encoded by this gene forms a heterodimer MutS beta with MSH2. MSH2-MSH3 heterodimer has a higher affinity for ≥ 2 base insertions/deletions, responsible for the recognition of mismatched bases. MutS beta initiates MMR by binding to a mismatch and then forming a complex with MutL alpha heterodimer [15]. In addition to its role in MMR, the MSH2-MSH3 complex is required in double strand break (DSB) [16]. The MutS beta complex suppresses chromosomal instability and modulate tumorigenesis due to its role in both DNA DSB repair and MMR [17]. Altered expression of MSH3 is also a common occurrence in a variety of cancers, including nonsmall cell lung cancer [18] and bladder cancer [19]. Altering the nuclear localization of hMSH3 disrupt DNA MMR in CRC cells [20]. Moreover, genetic variations in MSH3 were found to have an impact on the risk of sporadic CRC [21]. Furthermore, MSH3 polymorphisms were also predictors of patients’ clinical outcomes in CRC [22]. In our study, G allele carriers of rs12513549, A allele carriers of rs33013, and G allele carriers ofrs6151627, located in the intron area of MSH3, exhibited increased risk of grade ≥ 2 diarrhea. MSH3 rs12513549 is a G>T single nucleotide variation, rs33013 and rs6151627 are A>G single nucleotide variations. We speculated that the three SNPs (including rs12513549, rs33013, and rs6151627) may disrupt the capacity of MSH3 in DSB repair and MMR so that DNA damage caused by chemical agents and radiation accumulated or decreased, and finally resulting in the occurrence of grade ≥ 2 diarrhea. Furthermore, rs6151627 A>G genetic variation was shown to be associated with poor hematologic toxicity tolerance in non-small cell lung cancer patients after being treated with platinum-based chemotherapy, not associated with gastrointestinal toxicity [13]. The disparity may be partially explained by the different cancer types.
Like the major and well-described MMR genes, such as MSH2, MSH6, MLH1, and PMS2, the role of PMS1 in MMR system could not be underestimated. Briefly, MLH1 dimerizes with PMS1, PMS2, or MLH3 to form the MLH1/PMS2 (MutL alpha), MLH1/PMS1 (MutL beta), or MLH1/MLH3 (MutL gamma) heterodimer. MutL complex, cooperating with MutS complex, bind the DNA helix and recognize DNA mismatches [23]. Aberrant PMS1 expression was observed in thyroid cancer [24]. In addition to MSH2, MLH1, MSH6, and PMS2 those four genes, mutations in PMS1 have also been proposed to play a role in Lynch syndrome predisposition [25], an autosomal dominant condition (hereditary non-poly-posis colorectal cancer) which caused by MMR deficiency, typically presenting microsatellite instability. As previously reported, patients with CRC who were MMR deficient seem to have a predicted poor response to 5-fluorouracil–based adjuvant chemotherapy treatment [10,26]. MMR-deficient tumors were resistant to 5-fluorouracil–based adjuvant chemotherapy treatment, resulting from FU/A pairs, which was very closely resemble T/A pairs, escaping from mismatch repair, causing cells unable to detect DNA damage and promote cell apoptosis. In another hand, MMR increasing the mutation rate throughout the genome resulting in genomic instability is also an indirect reason [27,28]. An important finding in our current study was that PMS1 polymorphisms may affect the occurrence of dermatitis and prognosis in patients with rectal cancer. Previous studies have yet found that rs5742933 variation in PMS1 may serve as candidate prognostic markers of clinical outcome of non-small cell lung cancer[29]. Surprisingly, the associations between PMS1 genetic variations and prognosis of rectal cancer were observed for the first time. The underlying molecular mechanism for these polymorphisms is still unclear. It is speculated that genetic variations in PMS1 affected the risk of dermatitis and clinical outcome by disrupting the MMR system. We have previously examined the associations between MMR polymorphisms and CRT sensitivity and survival in patients with rectal cancer treated with preoperative CRT and found that SNPs in MLH3 and MSH2 genes had impact on CRT sensitivity and survival [30].
To the best of our knowledge, this is the first study to assess the effect of MMR polymorphisms on the risk of AEs and prognosis of locally advanced rectal cancer treated with total mesorectal excision surgery followed by concurrent CRT in Chinese populations. Moreover, patients were enrolled from the same center and follow-up data were collected by the same person, indicating that they were highly homogeneous for their ancestry, excluding the possible population stratifications. Nevertheless, our results should be validated in prospective studies with larger group of patients and comprehensive design and functional molecular mechanisms of these SNPs need to be studied further.
This study suggests that four SNPs (including rs12513549, rs33013, and rs6151627 in MSH3 and rs1233255 in PMS1) are associated with the risk of AEs, and three SNPs, including rs4920657, rs5743030 and rs5743100 in PMS1, are associated with survival of rectal cancer patients receiving postoperative CRT. Therefore, these polymorphisms may be potential independent biomarkers for predicting AEs and prognosis in rectal cancer patients receiving postoperative CRT.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by grants from National Basic Research Program of China (No. 2014CB542004 to W.T.), CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1-001 to W.T.), and National Natural Science Foundation (No. 81272510 to J.J.).