Laparoscopic Assisted Distal Rectal Cancer Resection with Preoperative Concurrent Chemoradiotherapy
Article information
Abstract
Purpose
Anatomy of deep pelvis, narrow distal margin and tumor invasion into neighbor organ are obstacles for curative radical resection for advanced cancer of distal rectum. Technically, laparoscopic application after downstaging the tumor with preoperative concurrent chemotherapy (CCRT) may give a solution to overcome the anatomical difficulties. We compared the results of laparoscopic surgery in the patients who received CCRT with those of patients who had conventional surgery.
Materials and Methods
A continuous infusion of 5FU plus leucovorin and radiotherapy (50.4 Gy) in 28 fractions was given each patient as CCRT. They underwent D2 radical resection with TME and ANP for the rectal cancer in 4 weeks.
Results
Thirty three patients had laparoscopic resection such as LAR, colo-anal anastomosis and APR. The results were compared with 12 cases of the conventional resections. As a result of preoperative CCRT, the cancer was down-staged in 71%. Two year disease free survival was 75% and 74% in the group of conventional and laparoscopic resection, respectively (p=0.427). Ileus, voiding difficulty and leakage after surgery were not different between two groups. Weakness of ejaculation was noted in 9~11% of both groups. The DFS of the preoperative CCRT followed by radical resection in the groups with a response was more favorable than that in the group with progressive or stable disease.
Conclusion
Radical resection of advanced distal rectal cancer could be done with performing a laparoscopic assisted operation after CCRT induced down-staging. We may suggest that laparoscopic assisted resection is a good treatment option as it doesn't increase the complications and it has a compatible survival rate to conventional surgery.
INTRODUCTION
The surgical management of distal rectal cancer requires radical resection of primary tumor, extensive lymph node dissection, anal sphincter preservation and the retention of sexual function. Total mesorectal excision and autonomic nerve preservation have received a great deal of attention by physicians. The characteristics of the pelvic structure, that is, the pelvis being narrow and deep frequently hinders smooth progress of an operation, and especially in male patients. The introduction of laparoscopy into rectal cancer surgery has helped to get the detailed anatomy due to both the magnified view and the near view with using a scope. It was easier to identify the Denonvillier's fascia, the pelvic nerve and the pelvic floor with performing laparoscopy than when performing conventional surgery. Laparoscopic total mesorectal excision with autonomic nerve preservation was reported to be feasible after analysis of cadaver models (1).
Yet there is very little of the tactile sense when performing laparoscopic surgery. It is hard to evaluate cancer invasion into the seminal vesicle, prostate and vaginal wall in patients with advanced distal rectal cancer. Preoperative concurrent chemoradiotherapy (CCRT) has been shown to downstage tumors and reduce the bulk of tumor, thereby allowing for a sphincter-preserving procedure (2,3). It also helps the surgical dissection for marginal cases. We suggest that the patients who had preoperative CCRT would be adequate candidates for laparoscopic resection, and even for cases with advanced rectal cancer.
In this study, we analyzed the complications and survival of the patients suffering with rectal cancer and who underwent laparoscopic radical resection after preoperative CCRT.
MATERIALS AND METHODS
1) Patients and groups
A total of 45 patients with advanced rectal cancer were treated, between Jan 2002 and Dec 2006 at the Hallym University Sungshim Hospital, with preoperative CCRT and radical rectal resection for their distal rectal cancer. The subjects were enrolled if they fulfilled the following eligibility criteria: (1) they had advanced rectal cancer with pathological documentation; (2) preoperative CCRT was fully received; (3) they had a WHO performance status of 3 or less; (4) there was no evidence of distant metastasis at the initial diagnosis; and (5) they had received no previous therapy. The patients were non-randomly allocated with the protocol. The data was analyzed retrospectively.
2) Treatment protocol
(1) CCRT; preoperative concurrent chemoradiotherapy
The eligible patients were treated as follows: 5FU, 325 mg/m2 and leucovorin 20 mg/m2 was given as an intravenous continuous infusion in 500 ml of saline over 6 hours on days 1~5 and days 29~33. The radiotherapy was delivered in the afternoon, after an infusion of chemotherapeutic agents, with total dose of 50.4 Gy in 28 fractions. Radical resection was then performed after four weeks.
(2) Radical rectal resection with conventional versus laparoscopic assisted surgery
We performed conventional radical surgery with a lower midline incision, high resection of the inferior mesenteric artery, D2 lymph node dissection, total mesorectal excision and autonomic nerve preservation. For the laparoscopic assisted radical surgery, we made five to six ports and we adhered to the principles of radical surgery with functional preservation as well (Fig. 1). The splenic flexure was mobilized for the laparoscopic assisted groups. Dissection around the origin of the inferior mesenteric vessels was done with using the medial to lateral approach. When a coloanal anastomosis with intersphincteric resection was required, then a planned temporary fecal diversion with loop ileostomy was performed, which was reduced in eight weeks or later.
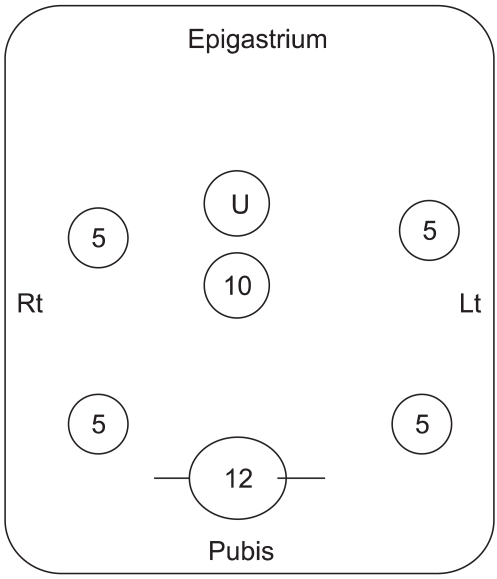
The laparoscopic ports for trocars were placed on the lower abdomen. Ten mm port for the scope was just below the umbilicus. The main transverse incision for removal of surgical specimen was on the line of 12 mm port in the supra-pubic area with 7 cm length or more. Four 5 mm ports were located lateral to the rectus abdominis muscle. U stands for umbilicus.
3) Assessment of the efficacy and complications
The effect of preoperative CCRT on rectal cancer treatment was evaluated with regard to the down staging and disease free survival (DFS). The response after CCRT was evaluated by comparing the tumor sizes in the pretreatment clinical T stage (cT) with the size in the post-CCRT stage (yT). The tumor sizes were measured with using the protocol of 3D-colon CT with reference to the RECIST criteria (4). The down-staging with response was defined as a 30% or more reduction in size of tumors, and stable-staging was defined as less than a 30% reduction or a 20% or less increase; progress-staging was defined as a more than a 20% increase. The laparoscopic resection group was compared with the conventional surgery group for determining the disease free survival and complications. The postoperative ileus was defined as no bowel gas passing within 72 hours after the operation with marked abdominal distension. Urinary voiding difficulty was defined as there was chronic urinary retention with residual urine in spite of three days of training and it was necessary to consult with the urologic department. Sexual disturbance in the male patients was evaluated with direct questions and answers at six months postoperatively. The complications at 30 days postoperatively were reviewed.
4) Statistics and survival
Statistical analyses were performed with chi-squared tests and Student's t-test, whenever appropriate, to compare the categorical variables between the groups. The disease survival curves were calculated by the Kaplan-Meier method. Differences in the survival curves were measured using log-rank tests. Statistical significance was defined as p-values less than 0.05.
RESULTS
1) Patient demographics and characteristics
Forty five of the 47 patients were analyzed. Two patients were excluded from the study because one patient had necrotizing fascitis perioperatively, and one patient was lost to follow up. The demographics of each group are listed in Table 1. The mean age of the patients was 57 years old. Most of the lesions were located in the lower rectum and mid rectum (64~66% and 30~34%, respectively). There were more male patients than females. Most of the lesions involved the perirectal fat (T3). Sphincter-saving operations were done in 51% and 81% of the conventional groups and laparoscopic ones, respectively.
2) Down staging after CCRT and the yT stage
Down-staging with a response was accomplished in 71% of the patients; stable disease was noted in 24% and progressive disease was noted in 5% with regarding to tumor size (Fig. 2). The median size of the cT was 5.2 cm (range: 2.3~10.5 cm) and that of the yT was 3.5 cm (range: 1.5~5.5 cm). For the pathologic staging (yT) after CCRT, 4% of all the irradiated tumor was in the T0 stage, 7% was in T1, 11% was in T2, 74% was in T3 and 4% was in T4 (Table 2). There were two cases of yT0, and these were diagnosed as cT4 with pretreatment 3D-CT. Because of splenic flexure mobilization, the non-irradiated colonic segment of the proximal sigmoid and descending colon was long enough to reach the pelvic floor in the laparoscopic assisted group. Colo-anal anastomosis was done without tension in the laparoscopy group. Colo-anal anastomosis and intersphinceric resection with or without a pouch was done in two of the 12 cases in the conventional surgery group and in six of the 24 cases in the laparoscopic surgery group.
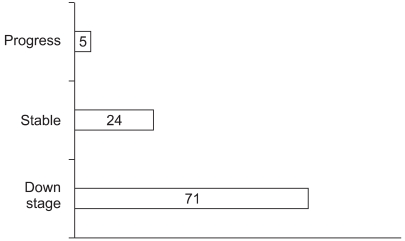
The result of down-staging after CCRT for the rectal cancer was showed along with RECIST criteria. yT stage was evaluated with 3D CT regarding size and radiologist reading. The PR and CR were in the cancers of seventy one percent of CCRT patients.
3) Postoperative complications (Table 3)
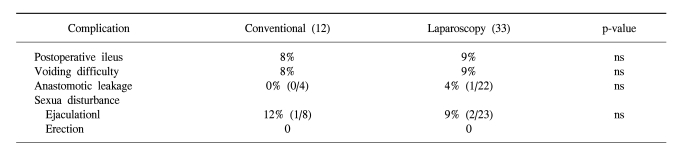
For the laparoscopic surgery group, the incidence of postoperative ileus was 9% and that of significant urinary voiding difficulty was 9%. One of the total laparoscopic low anterior resections had perioperaive anastomotic leakage (4%). There was no case of erectile sexual disturbance among the 23 males in both groups. There was no difference in the ejaculatory sexual dysfunction between the conventional and laparoscopic groups (12% vs 9%, respectively).
4) Follow up and disease free survival (DFS)
The median follow up was 26 months. There were four recurrences and metastasis for the conventional surgery group; one was regional, one was pelvic and lung metastasis, and two were lung metastasis. Seven recurrence and metastasis developed in the laparoscopic surgery group; two were regional, one was in the lung and liver, two were in liver, one was in bone and one was in a groin lymph node.
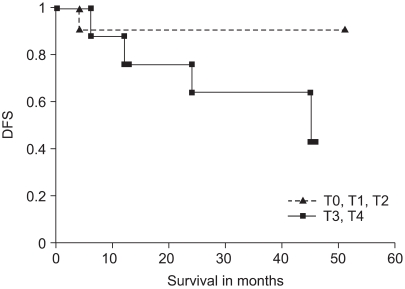
The overall disease free survival after preoperative CCRT and surgical resection was 83% and 68% at one year and three year, respectively (Fig. 3). The disease free survival of the laparoscopic group was not significantly different from that of the conventional radical resection group (Fig. 4, p=0.427). The disease free survival in the groups with down-staging with a response as yT1 and yT2 was favorable (Fig. 5 and 6), as compared to the non-response with CCRT group.
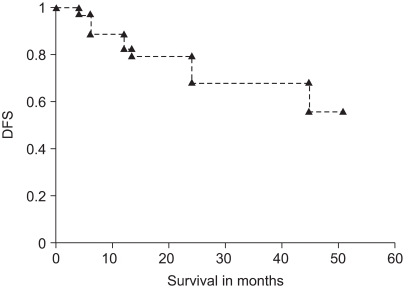
Disease free survival (DFS) in the rectal cancer patients (n=45) with preoperative CCRT followed by radical resection in 4 weeks was plotted. DFS was 83%, 68% in one year and three year, respectively.

Disease free survival (DFS) in conventional radical resection and laparoscopic radical resection for the rectal cancer after CCRT was plotted. The difference was not statistically significant between the two groups (p=0.427 with log rank test).

Disease free survival (DFS) of the preoperative CCRT followed by radical resection in the groups of response (n=32) was favorable than those of progress or stable (n=13). The difference was not statistically significant (p=0.348 with log rank test).
DISCUSSION
Preoperative assessment of rectal cancer patients allows individualized management strategies. For the distal rectal cancer patients, 80~90% of the patients have a choice between low anterior resection and abdominoperineal resection, and this choice depends on the tumor stage, the patient's body-figure, the surgeon and the preoperative management. Down staging and reduction of a tumor's bulk is important for patients to undergo radical surgery and sphincter preserving operation. Advocates of neoadjuvant chemoradiation or preoperative concurrent chemoradiotherapy give it with the intent to improve respectability, local control and possibly survival (5). T-level down staging after CCRT was reported to occur in 41% of patients with preoperative ultrasound stage T3/T4 disease (6). Conventional imaging with using CT and MRI shows only 60~80% and 55~95% accuracy of tumor staging, respectively (7). We did not compare the ultrasound stage because we could not frequently check the ultrasound stage due to lumen stricture. Rather, we measured the tumor size with performing 3D CT at the initial stage. The response was evaluated with reference to the RECIST classification at 4 weeks after the completion of CCRT. The tumor diameter was significantly decreased in 71% of all cases. The pathologic response was the criteria of a few reports such as Mandard's tumor regression grade, Dworak's tumor regression grade, the Japanese grading for therapeutic response and the criteria by the Korean GI Pathologic Study Group (8). Preoperative rectal biopsy and evaluation of the gene expression profiling for the effective response is a good tactic for selecting the preoperative CCRT patients (9,10). Gene expression profiling with using a human Gene Chip may be useful in predicting the response to radiotherapy to establish an individualized tailored therapy for rectal cancer. J-G Park or Watanabe reported on a predictive model with an accuracy of more than 80% (11).
Yet for performing surgical resection, the tumor size, location and invasion are the truly practical variables in the pelvis. The pathological complete response rate has usually been less than 20%. The response rate has varied in reports that had different follow up periods. If resection is impossible, then the rectal cancer cannot be cured. Even if the CCRT does not achieve complete regression, the decrease of tumor size itself helps the easy dissection for curative resection in addition to getting safe resection margins.
In our study, we performed radical rectal resection four weeks after CCRT. There was a previous report that a longer time interval between completion of neoadjuvant chemoradiation and the surgical resection did not improve down-staging of rectal carcinoma more than an interval of four to eight weeks (12).
Randomized trials such as the COLOR and COST trials suggested that laparoscopic colonic resection was one option for radical surgical treatment for colon cancer (13,14). We need greater experience and evidence that laparoscopic surgery is one of the standard treatments for advanced rectal cancer. Preoperative neoadjuvant chemoradiotherapy or CCRT not only provides improved disease free survival after resection, but this also significantly decreases the size of tumor, and it made laparoscopic resection possible for advanced rectal cancer.
As a result of animal experiments, we can speculate that the surgical intra-peritoneal microenvironment enhances successful implantation of spilled tumor cells, whereas the growth of adhered tumor cell clusters is not affected. The inflammatory response as a result of remote surgery also promotes successful tumor development (15). That is one of the theoretical reasons that minimal invasive surgery such as laparoscopy creates a less favorable environment for implantation of tumor.
As laparoscopic mobilization of the splenic flexure provided enough descending colon to reach the bottom of the pelvis, we removed the irradiated rectum and sigmoid, and this would be difficult in conventional surgery. Intersphincteric resection has recently offered more adequate resection margins than does low anterior resection. We expect that intersphincteric resection after CCRT increased the chances of sphincter preservation.
The laparoscopic access technique may cause specific complications that are associated with a laparoscopic approach, and especially on the bowel, bladder and vessels (16). But in this current series, laparoscopic surgery did not increase the postoperative complication such as ileus and voiding difficulty. Sexual disturbance in male patients was not increased as well. As for the other laparoscopic surgeries, they were beneficial for the early postoperative recovery such as the bowel passage and the minimal invasive wound in the abdomen. The pathogenesis of ileus involves inhibitory neural reflexes and inflammatory mediators from the site of injury (17).
Because of the short median survival and limited number of cases in our study, it was difficult to compare survival between the conventional surgery group and the laparoscopic one. Yet the disease free survival was not significantly different at 24 months postoperatively. We suggest that the CCRT-response group has a better prognosis and that this group is more suitable for laparoscopic surgery than the group with non-response to CCRT. Conducting a randomized trial for advanced rectal cancer after CCRT could determine if laparoscopic resection is as effective as conventional surgery and if it has benefits as a surgical technique to overcome the difficult pelvic anatomy.
CONCLUSIONS
Preoperative concurrent chemo-radiotherapy, CCRT, induced decrease of tumor size in 71% of the cancer patients of distal rectum. Laparoscopic assisted resection in these patients was safe and effective as same as conventional radical resection with regards to disease free survival and complications.