Intercalated Treatment Following Rebiopsy Is Associated with a Shorter Progression-Free Survival of Osimertinib Treatment
Article information
Abstract
Purpose
Epidermal growth factor receptor (EGFR) T790M mutation serves as an important predictor of osimertinib efficacy. However, little is known about how it works among patients with various timings of T790M emergence and treatment.
Materials and Methods
Advanced EGFR-mutant lung adenocarcinoma patients with positive T790M mutation in tumor were retrospectively enrolled and observed to determine the outcomes of osimertinib treatment. We evaluated the association between patients’ characteristics and the efficacy of osimertinib treatment, particularly with respect to the timing of T790M emergence and osimertinib prescription.
Results
A total of 91 patients were enrolled, including 14 (15.4%) with primary and 77 (84.6%) with acquired T790M mutation. The objective response rate and disease controlratewere 60.9% and 85.1%, respectively. The median progression-free survival (PFS) and overall survival were 11.5 months (95% confidence interval [CI], 9.0 to 14.0) and 30.4 months (95% CI, 11.3 to 49.5), respectively. There was no significant difference in response rate and PFS between primary and acquired T790M populations. In the acquired T790M subgroup, patientswho received osimertinib after T790M had been confirmed by rebiopsy had a longer PFS than those with intercalated treatments between rebiopsy and osimertinib prescription (14.0 months [95% CI, 9.0 to 18.9] vs. 7.2 months [95% CI, 3.7 to 10.8]; adjusted hazard ratio, 0.48 [95% CI, 0.24 to 0.98; p=0.043]). Rebiopsy timing did not influence the outcome.
Conclusion
Osimertinib prescription with intercalated treatment following rebiopsy but not the timing of T790M emergence influenced the treatment outcome. We suggest that it is better to start osimertinib treatment once T790M mutation has been confirmed by biopsy.
Introduction
Epidermal growth factor receptor (EGFR) mutation is one of the most common oncogenic drivers in lung cancer patients [1,2]. Since EGFR–tyrosine kinase inhibitor (TKI) can offer a better efficacy and quality of life [3,4], it has emerged as an important first line therapy in EGFR-mutant non-small cell lung cancer (NSCLC) patients. Although most EGFR-mutant NSCLC patients may experience a good response to EGFR-TKI initially, acquired resistance inevitably occurs, which leads to a progression of the disease [5]. The most common mechanism of acquired resistance is a secondary EGFR mutation that involves a substitution of threonine to methionine at codon 790 (T790M) [6].
In our previous study [7], we suggested that the detection of T790M should not be limited at the time of EGFR-TKI progression because it could also be identified among patients experiencing an interval from progression to initial EGFR-TKI treatment as well as among those lacking the continuing EGFR-TKI treatment at the time of rebiopsy. Furthermore, a small portion of EGFR-mutant patients were known to harbor primary T790M before EGFR-TKI treatment [2]. Although previous studies suggested that T790M mutation has a “wax and wane” nature [8], little is known about the true dynamics of T790M mutation and how it interferes with patients’ outcomes and treatment results.
Osimertinib is a third-generation EGFR-TKI that selectively targets both sensitizing EGFR mutations and T790M resistance mutation. Several clinical trials have shown that it exhibits promising efficacy and has a more favorable adverse events profile for EGFR-mutant patients resistant to prior EGFR-TKI therapy [9-11], and T790M was found to serve as a key predictor of the efficacy. Clinical trials enrolled patients with T790M mutation detected after disease progression on “the last treatment regimen” [9,11]. However, real-world patients may not always have the chance to receive osimertinib treatment immediately after T790M confirmed by rebiopsy. Currently, it remains unclear whether treatment efficacy varies among patients with different intervals between T790M emergence and osimertinib prescription. We conducted this study to evaluate the association between patients’ characteristics and the efficacy of osimertinib treatment, with a particular emphasis on both the timing of T790M emergence and osimertinib prescription.
Materials and Methods
1. Patients
We retrospectively analyzed lung cancer patients who were diagnosed and treated with osimertinib at Taichung Veterans General Hospital (TCVGH) between September 2014 and January 2017. To be eligible for participation in the study, patients were required to have pathologically confirmed lung adenocarcinoma, advanced detection spectrum of MALDI- (stage IIIB and IV) disease, known sensitive EGFR mutations in treatmentnaïve tumor specimens, positive T790M mutation in tumor tissue (either primary or acquired after EGFR-TKI progression), a history of osimertinib therapy, and complete clinical follow-up data. Patients were excluded if they had lung tumor with doubtful origin, other active malignancies, T790M detected only in plasma ctDNA, incomplete data records, or received other anti-neoplasm therapy during the course of osimertinib treatment (e.g., chemotherapy and immunotherapy).
2. Data records and response evaluation
Clinical data for analysis included patients’ age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage, smoking status, EGFR mutation status, biopsy condition, and serial treatment history. Brain metastasis status at the time of osimertinib treatment was also recorded. Tumor, node, and metastases (TNM) staging was done according to the seventh edition of the American Joint Committee for Cancer staging system [12]. Unidimensional measurements as defined by Response Evaluation Criteria in Solid Tumors ver. 1.1 were used in this study [13]. The objective of this study was to compare the efficacy of osimertinib in T790M-positive lung adenocarcinoma patients with various treatment and biopsy conditions. Outcome variables included the objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
3. EGFR mutation analysis
Tumor specimens were collected and procured for EGFR mutation analysis as previously described [14]. The detection method used in this study was matrix-assisted-laser-desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). The detection spectrum of MALDI-TOF MS is summarized in S1 Table. We performed the testing according to the instructions provided by the MassARRAY system (Sequenom, San Diego, CA). With respect to the biochemical reaction, polymerase chain reaction was used to amplify the region containing the tyrosine kinase domain of the EGFR exons 18, 19, 20, and 21. A single nucleotide extension was then performed by primers and corresponding detection probes to amplify the region containing each target mutation. After SpectroClean Resin clean up, samples were loaded onto the matrix of SpectroCHIP by Nanodispenser (Matrix) and then analyzed by Bruker Autoflex MALDI-TOF MS. Data were collected and analyzed by Typer4 software (Sequenom). All the tests were performed by ISO15189-certified TR6 Pharmacogenomics Lab, National Research Program for Biopharmaceuticals (NRPB), at the National Center of Excellence for Clinical Trial and Research of National Taiwan University Hospital.
4. Statistical methods
With regard to the rebiopsy timing, patients who had received other systemic treatment between the first EGFR-TKI progression and rebiopsy were defined as rebiopsy “with interval from first EGFR-TKI progression” and patients receiving rebiopsy at the time of first EGFR-TKI progression were indicated as rebiopsy “at first EGFR-TKI progression.” Patients who had continued receiving EGFR-TKIs treatment within 1 month before rebiopsy, were defined as “with EGFR-TKI treatment at rebiopsy.” With regard to the treatment timing, patients who had received any other systemic treatment, such as chemotherapy, immunotherapy, and other targeted therapy, between rebiopsy and the prescription of osimertinib were defined as osimertinib treatment “with intercalated treatment” and patients without intercalated treatments between rebiopsy and osimertinib prescription were indicated as osimertinib treatment “after rebiopsy.” We also evaluated the interim between rebiopsy and osimertinib treatment (< 6 months vs. ≥ 6 months). Univariate analyses of ORR and DCR were performed using Fisher’s exact test. The Kaplan-Meier method was used to estimate PFS and OS. Differences in survival time were analyzed by log-rank test. Logistic regression model and Cox proportional hazard model were used for multivariate analyses of treatment responses and survival outcomes. In the stepwise procedure, the significant levels for entry and removal were 0.05 and 0.10, respectively. All statistical tests were carried out using SPSS ver. 15.0 (SPSS Inc., Chicago, IL). Two-tailed tests and p-values of < 0.05 for significance were used.
5. Ethical statement
This study was approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB No. CF12019). Written informed consents for genetic testing and clinical data records were obtained from all patients.
Results
1. Patient demographics and osimertinib treatment
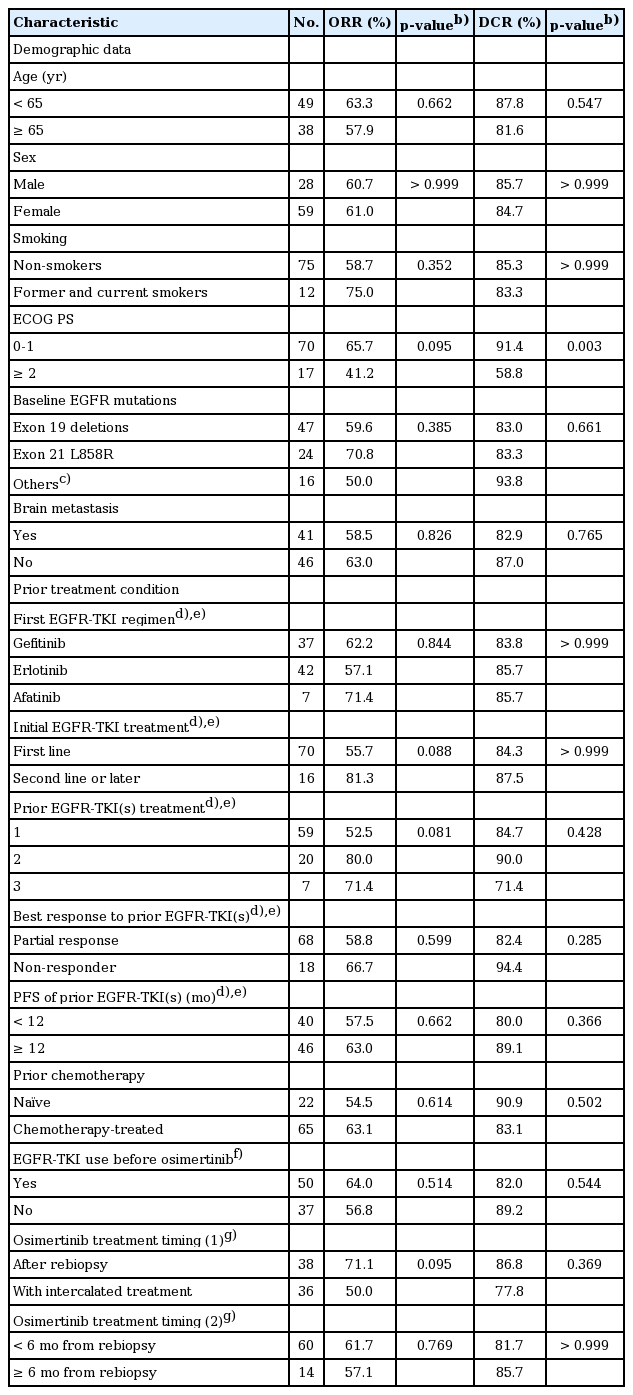
The selection flowchart of the study population is shown in Fig. 1. A total of 91 patients with advanced lung adenocarcinoma harboring T790M and treated with osimertinib were enrolled for outcome analysis. Patients’ characteristics are shown in S2 Table. Briefly, the median age was 63 years. Sixty-one patients (67.0%) were female and 77 patients (84.6%) were non-smokers. Most patients (79.1%) received EGFR-TKI as the first line therapy. Sixty-three patients (69.2%) received one EGFR-TKI treatment previously and 27 patients (29.7%) had received two or more prior EGFR-TKIs. One patient with primary T790M did not receive first or second generation EGFR-TKI before osimertinib treatment. Seventy-three patients (80.2%) had ECOG PS 0-1 and 22 patients (24.2%) were chemonaïve. Forty-one patients (45.1%) had brain metastasis at the time of osimertinib treatment. Exon 19 deletion (19Del) and exon 21 L858R accounted for the most common baseline EGFR mutation types (52.7% and 27.5%, respectively). Other mutations included one with G719S, one with G719S+S768I, two with 19Del+G719X, and 14 with primary T790M (4 accompanied with 19Del and 10 accompanied with L858R).
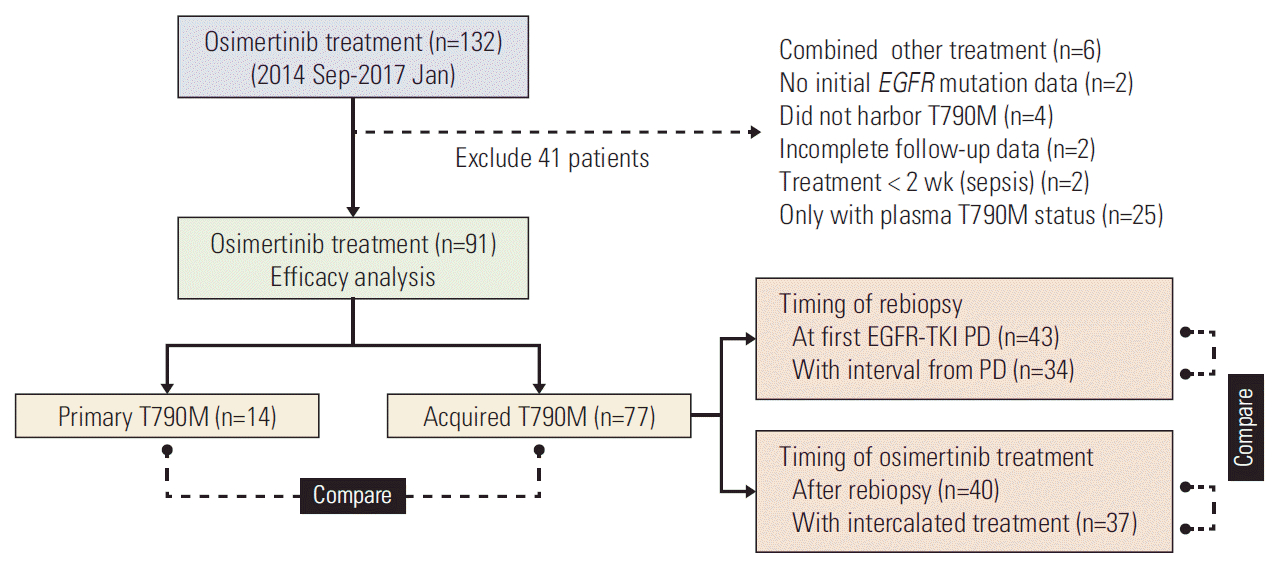
Patient selection flowchart. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PD, disease progression.
After exclusion of four patients without a measurable lesion, the ORR and DCR were 60.9% and 85.1%, respectively. Survival outcomes were followed up till June 30, 2017. The median PFS and OS were 11.5 months (95% confidence interval [CI], 9.0 to 14.0) and 30.4 months (95% CI, 11.3 to 49.5), respectively. With regard to the timing of T790M emergence, 14 patients (15.4%) harbored primary T790M and 77 patients (84.6%) had acquired T790M after EGFR-TKI treatment. Among the acquired T790M population, rebiopsy was performed at first EGFR-TKI progression in 43 patients and the other 34 patients received rebiopsy following an interval from the first EGFR-TKI progression. In the case of treatment timing, 40 patients received osimertinib after T790M was confirmed by rebiopsy and the other 37 patients had intercalated treatment between rebiopsy and osimertinib prescription.
2. Osimertinib efficacy and patients’ clinical condition
Univariate analyses of the best response of osimertinib are shown in Tables 1 and 2. In the ORR analysis, no factor correlated significantly with objective response to osimertinib treatment. There were trends of differences in ECOG PS, EGFR-TKI as the first line treatment or not, numbers of prior EGFR-TKI treatments, osimertinib treatment timing, and the rebiopsy timing of acquired T790M. In the DCR analysis, ECOG PS was the only factor that correlated with disease control significantly. Patients with ECOG PS 0-1 were more likely to achieve disease control (91.4% vs. 58.8%, p=0.003).
For the overall population, no covariate reached a significant level in the multivariate logistic regression model for ORR analysis. In the case of DCR analysis, only ECOG PS 0-1 independently correlated with a higher chance of disease control (odds ratio [OR], 9.00; 95% CI, 2.35 to 34.54; p=0.001). In the multivariate Cox proportional hazard model, ECOG 0-1 was the only factor associated with both a longer PFS and OS independently (hazard ratio [HR], 0.32; 95% CI, 0.16 to 0.63; p=0.001 and 0.21; 95% CI, 0.09 to 0.49; p < 0.001, respectively).
3. Osimertinib among patients with primary or acquired T790M
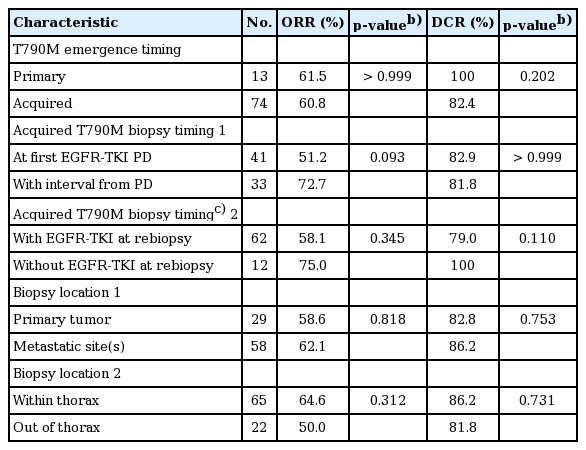
As shown in Table 2, the ORR and DCR among patients with primary and acquired T790M were 61.5% vs. 60.8% and 100% vs. 82.4%, respectively. Neither p-values were statistically significant. S3 Fig. illustrates the PFS and a trend toward a longer PFS in primary T790M than in acquired T790M population was found (not reached vs. 10.7 months [95% CI, 8.0 to 13.5], p=0.093). Of the primary T790M patients, 11 were still continuing osimertinib treatment at the time of data cut-off and seven of them experienced more than 10 months’ PFS. OS was similar between each group (p=0.990).
The results of the multivariate analysis of ORR and PFS regarding the timing of T790M emergence are shown in Table 3. There was no significant difference in ORR (adjusted OR, 1.72; 95% CI, 0.25 to 11.93; p=0.581) and PFS (adjusted HR, 0.60; 95% CI, 0.12 to 2.98; p=0.532) between each group.
4. Rebiopsy timing and osimertinib efficacy among patients with acquired T790M
With regard to the rebiopsy timing, we examined the interval between rebiopsy and “first” EGFR-TKI progression because EGFR-mutant patients usually benefit most from the first EGFR-TKI therapy but not the rechallenge [15]. As shown in Table 2, in the acquired T790M subgroup, the ORR and DCR among patients whose T790M detected at first EGFR-TKI progression and after an interval were 51.2% vs. 72.7% and 82.9% vs. 81.8%, respectively. Both p-values were not statistically significant. There were also no significant differences in PFS (11.5 months [95% CI, 5.1 to 17.8] vs. 9.2 months [95% CI, 7.5 to 11.0], p=0.700) (Fig. 2). Similar results were noted in the analysis of whether patients remained EGFR-TKI at the time of rebiopsy.
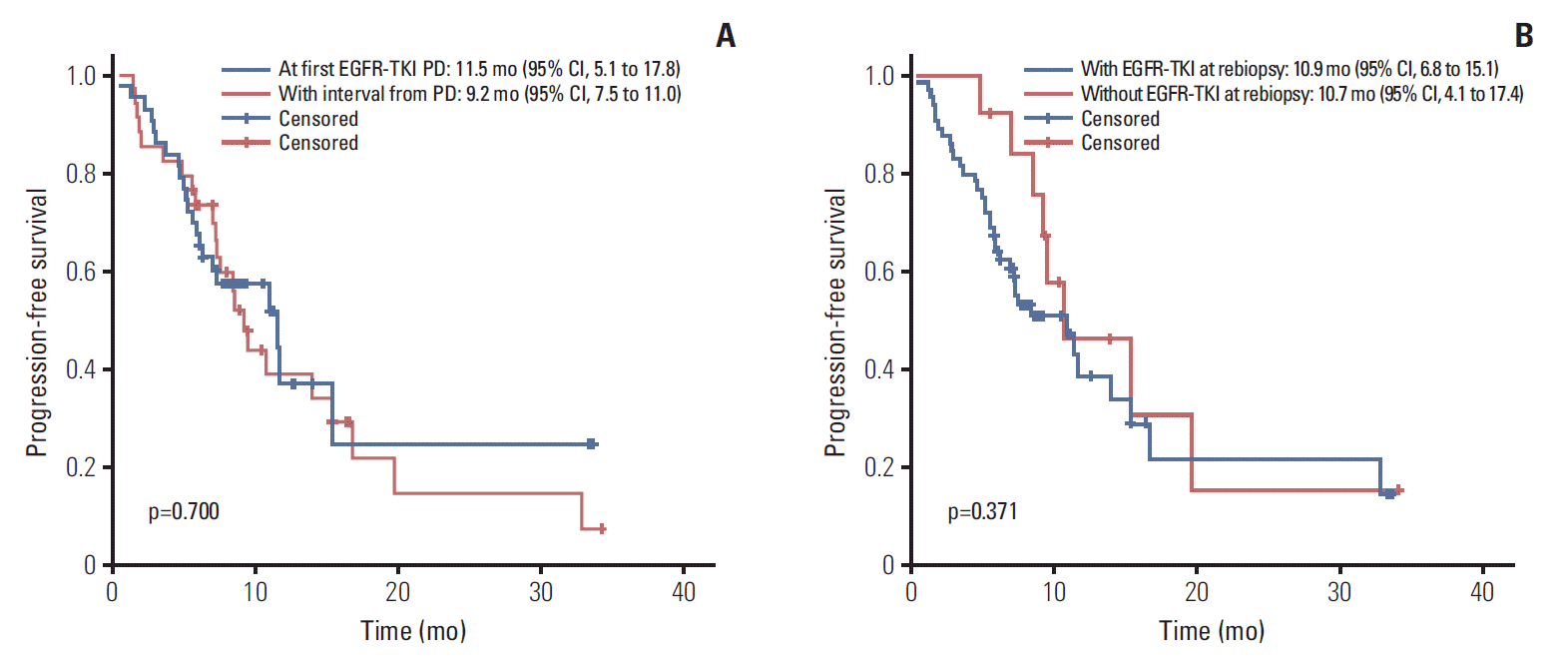
Kaplan-Meier plot showing progression-free survival of patients with acquired T790M in relation to rebiopsy timing (p-value by log-rank test). EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PD, disease progression; CI, confidence interval.
In the multivariate analysis (Table 3), there was no significant difference in ORR (adjusted OR, 0.69; 95% CI, 0.16 to 3.07; p=0.627) and PFS (adjusted HR, 0.97; 95% CI, 0.44 to 2.15; p=0.943) between patients whose T790M were detected at the first EGFR-TKI progression and after an interval. Similar results were noted in the analysis of whether patients were kept on EGFR-TKI at the time of rebiopsy.
5. Treatment timing and osimertinib efficacy among patients with acquired T790M
In the acquired T790M subgroup as shown in Table 1, the ORR and DCR among patients who received osimertinib after T790M was confirmed by rebiopsy and with intercalated treatment were 71.1% versus 50.0% (p=0.095) and 86.8% versus 77.8% (p=0.369), respectively. Similar results were noted in the analysis of whether patients started osimertinib treatment within 6 months after rebiopsy or not. With regard to the osimertinib treatment timing, there was no significant difference in ORR by the multivariate analysis (Table 3).
In the PFS analysis as shown in Fig. 3, patients who received osimertinib immediately after T790M was confirmed by rebiopsy and who started treatment within 6 months after rebiopsy had a longer survival time (14.0 months; 95% CI, 9.0 to 18.9 vs. 7.2 months; 95% CI, 3.7 to 10.8; p=0.008 and 11.7 months; 95% CI, 6.2 to 17.2 vs. 7.0 months; 95% CI, 4.6 to 9.4; p=0.012, respectively). In the multivariate analysis (Table 3), osimertinib treatment started after T790M was confirmed by rebiopsy and within 6 months after rebiopsy significantly correlated with PFS (adjusted HR, 0.48; 95% CI, 0.24 to 0.98; p=0.043 and 0.48; 95% CI, 0.24 to 0.97; p=0.040, respectively).
Discussion
The success of EGFR-targeted therapy has led to an era of precision medicine in lung cancer [16]. By now, personalized therapy has moved in the direction of the genotypic evolution of lung cancer, because the most common mechanism of resistance, the T790M mutation, could be overcome by treatment with third generation EGFR-TKI. Among patients who progress after first or second generation EGFR-TKI, T790M is not only a mechanism of resistance, but also serves as an important biomarker of subsequent osimertinib treatment [10,17]. The outcome of osimertinib treatment among T790M-positive patients in our study was comparable with that of previous clinical trials [9,11]. However, it should be noted that our cohort consisted entirely of ethnically Asian patients, who were more heavily treated, and there were greater prevalence rates of subjects with brain metastasis and poor performance status. Our results disclosed that ECOG PS remained an important prognostic factor, even in the setting of targeted therapy. Moreover, our findings indicated that the timing of treatment, but not the timing of T790M emergence, may significantly affect the efficacy of osimertinib treatment.
Because previous clinical trials focused on EGFR-TKI‒pretreated T790M-positive patients [9-11,17], little is known about the efficacy of osimertinib in patients harboring primary T790M. A study by Hata et al. [18] suggested that T790M could both pre-exist and evolve from the drug-tolerant cells, and that different mechanisms may result in distinct efficacy of treatment. Of them, tumors with pre-exist T790M may be more responsive to third generation EGFR-TKI. In the present study, there were 14 patients with primary T790M. All of them had concomitant sensitizing and T790M mutation in treatment-naïve tumor specimens (4 with 19Del and 10 with L858R). Although the overall efficacy was similar to that of acquired T790M patients, we observed a trend toward a longer PFS in patients with primary T790M (p=0.093) and 11 of them were still continuing osimertinib treatment at the time of data cut-off. Hence, we suggest that osimertinib might offer at least similar benefits to patients with primary T790M. A longer follow-up time and prospective studies enrolling more patients are needed to clarify the true efficacy of osimertinib in a primary T790M population.
Since previous studies suggested that primary and acquired T790M mutation had distinct preferences of concomitant mutation partners, different prognostic meanings, and potentially a different pathogenesis [18-21], they may represent two distinct entities. We performed a subgroup analysis of the acquired T790M population. Our previous study suggested that T790M could be identified not only at the time of EGFR-TKI progression or with the continuing EGFR-TKI treatment at the time of rebiopsy, but also in patients with intercalated treatment after EGFR-TKI progression [7]; herein, we further demonstrated that rebiopsy timing did not influence the efficacy of osimertinib. The results were similar with those of AURA and AURA2 studies [9,11], showing that the efficacy of osimertinib was consistent across subgroups with various last treatment regimens, in which the T790M was identified. The aforementioned data suggest that T790M might carry a significant oncogenic activity per se; hence, whenever it is detected, patients could benefit from anti-T790M treatment. For patients without suitable lesions for rebiopsy at the time of EGFR-TKI progression, an attempt to perform rebiopsy should be considered during the subsequent treatment courses [7].
Both AURA and AURA2 studies enrolled patients with positive T790M detected after progression of the last treatment [9,11]. ASTRIS (Real World Treatment Study of AZD-9291 for Advanced/Metastatic EGFR T790M Mutation NSCLC, NCT02474355) is an ongoing phase III study designed to assess the efficacy and safety of osimertinib in a real world setting [22]. However, only T790M mutation confirmed after last treatment progression is accepted for participation. In the present study, we further analyzed the interval between rebiopsy and osimertinib prescription and found that intercalation of other systemic therapy and length of the interval influenced the outcome of subsequent osimertinib treatment. Although ORR and DCR were not statistically significant in the analysis of treatment timing, patients with intercalated treatments or who started treatment more than 6 months after rebiopsy had a significantly shorter PFS. Maemondo et al. [23] compared the efficacy of gefitinib and chemotherapy for EGFR-mutant NSCLC patients in a randomized phase III study, and demonstrated that the ORR of gefitinib in first line and second line settings were 73.7% and 58.5%, respectively. Similar results were observed in a phase II study by Sugio et al. [24], and the PFS of the overall population, 7.1 months, was shorter than that observed in a pure treatment-naïve population [16]. Moreover, in the case of ALK mutation, a different efficacy of crizotinib could be observed between treatment-naïve and chemotherapyrefractory populations (PFS, 10.9 months vs. 7.7 months, respectively) [25,26]. These results imply that a complicated interaction exists between treatments. Because both the sensitizing EGFR mutation and T790M frequency are dynamic and would be diminished by the effective treatment following EGFR-TKI progression using quantitative method [27], the T790M level at the time of osimertinib prescription might be different in patients with or without intercalated treatment, which could explain at least partly the distinct outcome in this study. The underlying mechanisms of cancer evolution during the path of treatments require further investigation. Herein, we suggest that it is better to start osimertinib treatment once T790M has been confirmed by rebiopsy.
Liquid biopsy using plasma ctDNA, which is more convenient and carries lower risks, is an alternative method to obtain genetic information about tumors [28]. As compared with sensitizing mutations, the detection of T790M in plasma is associated with a lower sensitivity and specificity [29], which resulted in a different outcome of osimertinib treatment. Therefore, we did not include patients whose T790M mutation was only detected in plasma to avoid the potentially confounding variables. Our previous study showed that dynamic plasma EGFR mutation status can serve as an independent outcome predictor of EGFR-TKI therapy [30]. Theoretically, this concept can be applied in T790M detection and osimertinib therapy, too. Better platforms of liquid biopsy are needed to set up for clinical application.
The major limitation of the present study is the retrospective nature of this investigation. Although data were collected retrospectively, we tried to ensure the validity of patients’ characteristics, as well as the correlation between treatment course and outcome measurement. Primary T790M accounted for 15.4% of the study cohort but this did not represent the true prevalence of the whole population [2]. There were only a limited number of available cases involving rare mutations with T790M in our study and in clinical trials [9,11]; hence, the efficacy of osimertinib among this subgroup remains unknown. Moreover, further studies are needed to evaluate the dynamics and pathogenesis of T790M mutation during the course of lung cancer treatment.
In conclusion, patients with primary T790M could also benefit from osimertinib treatment and the efficacy was at least similar to that of acquired T790M. The results of our analysis of the acquired T790M patients suggest that the timing of treatment, but not the timing of rebiopsy, influenced the outcome of osimertinib treatment.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
We would like to thank the Comprehensive Cancer Center of Taichung Veterans General Hospital for assisting with data collection and management and we also thank the NRPB Pharmacogenomics Lab and the NCFPB Integrated Core Facility for Functional Genomics for their technical support.