The Prognostic Impact of the Number of Metastatic Lymph Nodes and a New Prognostic Scoring System for Recurrence in Early-Stage Cervical Cancer with High Risk Factors: A Multicenter Cohort Study (KROG 15-04)
Article information
Abstract
Purpose
We aimed to assess prognostic value of metastatic pelvic lymph node (mPLN) in early-stage cervical cancer treated with radical surgery followed by postoperative chemoradiotherapy. Also, we sought to define a high-risk group using prognosticators for recurrence.
Materials and Methods
A multicenter retrospective study was conducted using the data from 13 Korean institutions from 2000 to 2010. A total of 249 IB-IIA patients with high-risk factors were included. We evaluated distant metastasis-free survival (DMFS) and disease-free survival (DFS) in relation to clinicopathologic factors including pNstage, number of mPLN, lymph node (LN)ratio (number of positive LN/number of harvested LN), and log odds of mPLNs (log(number of positive LN+0.5/number of negative LN+0.5)).
Results
In univariate analysis, histology (squamous cell carcinoma [SqCC] vs. others), lymphovascular invasion (LVI), number of mPLNs (≤ 3 vs. > 3), LN ratio (≤ 17% vs. > 17%), and log odds of mPLNs (≤ ‒0.58 vs. > ‒0.58) were significant prognosticators for DMFS and DFS. Resection margin involvement only affected DFS. No significant survival difference was observed between pN0 patients and patients with 1-3 mPLNs. Multivariate analysis revealed that mPLN > 3, LVI, and non-SqCC were unfavorable index for both DMFS (p < 0.001, p=0.020, and p=0.031, respectively) and DFS (p < 0.001, p=0.017, and p=0.001, respectively). A scoring system using these three factors predicts risk of recurrence with relatively high concordance index (DMFS, 0.69; DFS, 0.71).
Conclusion
mPLN > 3 in early-stage cervical cancer affects DMFS and DFS. A scoring system using mPLNs > 3, LVI, and non-SqCC could stratify risk groups of recurrence in surgically resected early-stage cervix cancer with high-risk factors.
Introduction
Cervical cancer is newly diagnosed in 3,500 patients in the Korea in 2014 [1]. Despite a declining trend over the last 40 years, the incidence and mortality rate among young women increased [2]. Since cancer screening using Pap smear has been widely used, about three quarters of newly diagnosed cases are the International Federation of Gynecology and Obstetrics (FIGO) stage I or IIA [3]. These early cervical cancer are subject to radical surgery and has a relatively favorable prognosis with a 5-year overall survival (OS) rate of 90% and a disease-free survival (DFS) rate of 82% [4]. However, pathologic findings including positive resection margin (RM), parametrial (PM) involvement, and lymph node (LN) metastasis are considered as high risk factors for recurrence and patients with those factors are candidate for postoperative chemoradiotherapy (CRT) [5]. Pelvic LN metastasis is found in nearly half of cases, even in early stage cervical cancer [6] and in this setting, the 5-year relative survival rate was 57.4% [7]. In a Gynecologic Oncology Group (GOG) 49 study including FIGO IB patients, the 5-year DFS of patients with metastatic pelvic LN (mPLN) was 10% lower than those without [8]. In pooled analysis of GOG 24/56/59, LN metastasis was statistically significant variable for both DFS (hazard ratio [HR], 1.9) and OS (HR, 2.0) [9].
Several researchers have assessed prognostic meaning of mPLN in various ways. The simplest is counting the number of mPLN, which is intuitive and widely used in other cancers [10,11]. The LN ratio (LNR) which has been developed to reflect the extent of LN resection and the log odds of positive LN (LODDs) which has the advantage of reflecting the number of negative LNs are suggested as prognostic variables related to LN status [12]. However, those studies were limited in that most of them are single institutional retrospective series and also, included heterogeneous population in terms of tumor stages and treatment characteristics. Until now, it is unclear that among several parameters related to LN status, which one serves as a best prognostic factor.
We therefore aimed to assess prognostic value of the number of and related parameters of mPLN in patients with early-stage cervical cancer treated with radical surgery followed by postoperative CRT due to pathologic high risk factors. Also, we sought to find a high risk group of recurrence in these patients.
Materials and Methods
1. Patient cohort
We conducted a multicenter retrospective study (Korean Radiation Oncology Group [KROG] 15-04) at affiliated 13 institutions in South Korea. After the approval of Institutional Review Board of each institution, medical records of patients who were newly diagnosed of FIGO IB-IIA cervical cancer between 2000 and 2010, and treated with radical surgery followed by postoperative CRT due to pathologic high risk features including positive RM, PM invasion, and LN metastasis were reviewed. We intended to enroll homogeneous group of patients without clinically involved LN on preoperative positron emission tomography/computed tomography (PET/CT) or magnetic resonance imaging (MRI). This is because definitive CRT or radiotherapy (RT) is often recommended in those with gross mPLNs which are LNs with increased metabolism than the background (PET/CT) or those with a short diameter greater than 1 cm (MRI). Additionally, patients who received preoperative chemotherapy (CTx), who did not receive radical surgery, who have insufficient information on the extent of surgery or pathologic report, who did not complete adjuvant CRT, or who have with history of other malignancies were excluded. Finally, a total of 259 patients were candidates of this study, and each institutional review board’s approval was achieved. The patient selection process is depicted in S1 Fig.
2. Treatment
All patients underwent radical hysterectomy with pelvic LN dissection according to our inclusion criteria. Para-aortic LN sampling or dissection was carried out in 120 patients (46.3%). Postoperative RT was delivered to whole pelvis in daily fraction of 1.8-2 Gy with median dose of 50.4 Gy (range, 41.4 to 60.4 Gy). Brachytherapy was performed in 36 patients (18%) with median dose of 18 Gy (range, 5 to 28 Gy) following whole pelvic RT. Nineteen patients (7.3%) underwent para-aortic nodal irradiation with a median dose of 45 Gy (range, 45 to 60 Gy), of whom nine patients received RT prophylactically. Most of all patients (97.3%) received platinumbased CTx during CRT. The most common CTx regimen was weekly cisplatin (50.2%), and doublet of 5-fluorouracil and cisplatin was the second (24.5%).
3. Variables and statistical analysis
All patients were staged using 2009 FIGO staging system [13]. As FIGO staging system does not describe presence of LN metastases, pathologic nodal status was described separately as pN0 or pN1 based on seventh edition of American Joint Committee on Cancer staging system [14]. Additionally, we evaluated parameters related to LN status such as the number of mPLNs, LNR, and LODDs. LNR was calculated by dividing the number of mPLNs by the number of harvested LNs (Eq. 1).
LODDs was defined as the logarithm of odds ratio of negative LNs over positive LNs (Eq. 2) [12].
In this Eq. (2), 0.5 is added constant to avoid an infinite number. Cutoff value of these parameters was determined by maximal chi-square method.
Distant metastasis-free survival (DMFS) was defined as an interval between the surgery and the detection of distant metastasis or last follow-up. DFS was calculated from the surgery to the detection of any recurrence or last follow-up. In-pelvic recurrences are regarded as locoregional failures while para-aortic LN failures as distant. Survival curves were estimated by Kaplan-Meier method and survival differences were evaluated using log-rank test. Statistically significant variables in univariate analyses were included in a step-wise Cox proportional hazard regression model for multivariate analyses. In the modeling process, variables with p < 0.20 were put into and those with p > 0.10 were eliminated from the regression model. A p-values < 0.05 was considered statistically significant. All statistical analyses were performed by R ver. 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
4. Ethical statement
The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1511/324-109) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived.
Results
1. Patient demographics
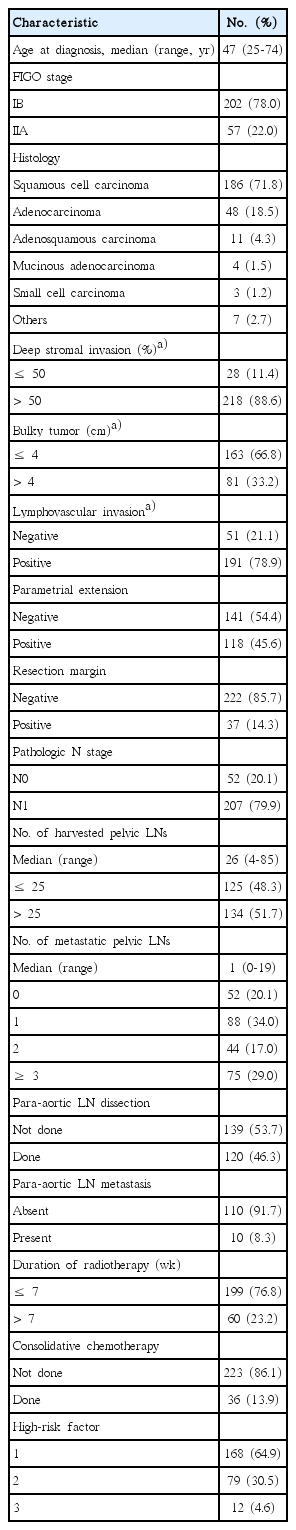
The median age of patients was 47 years (range, 25 to 74 years) and about three-quarters of patients was FIGO stage IB. The most common histology was squamous cell carcinoma (SqCC; 71.8%) followed by adenocarcinoma (ADC; 18.5%). Deep stromal invasion greater than 1/2 thickness, bulky tumor more than 4 cm, and lymphovascular invasion (LVI) were found in 88.6%, 33.2%, and 78.9% of patients, respectively. Patients with PM extension or positive RM were 45.6% and 14.3%, respectively. Two hundred and seven patients (79.9%) were pN1. The median number of harvested LNs and mPLNs were 26 (range, 4 to 85) and 1 (range, 0 to 19), respectively. Of 120 patients who underwent para-aortic LN sampling or dissection, 10 patients (3.9%) had metastatic para-aortic LNs. Thirty-six patients (13.9%) received a consolidative CTx following adjuvant CRT at the discretion of treating physician. Patient demographics are summarized in Table 1.
2. Survival analysis
As the presence of para-aortic LN metastases is an obvious poor prognostic factor for DMFS and DFS, 10 patients with para-aortic LN metastases were excluded from subsequent survival analyses to avoid possible confounding effect. Consequently, 249 patients were included for survival analyses of DMFS and DFS. Median follow-up duration was 70 months (range, 2.5 to 468.5 months). Five-year DMFS and DFS of 249 patients were 83.5% and 78.1%, respectively. Distant failure occurred in 42 patients and crude distant failure rate was 16.9%. The lungs were the most common site of failure (33.3%) and the second most common was para-aortic LN (28.6%). Locoregional failure occurred in 23 patients (9.2%) and there were 11 patients who experienced both locoregional and distant failure.
Determined cutoff value for the number of mPLNs, LNR and LODDs for DMFS were 3, 17%, and ‒0.58, respectively (S2 Fig.). The proportion of patients with mPLNs ≤ 3, LNR ≤ 17%, and LODDs ≤ ‒0.58 were 81.1%, 86.0%, and 87.6%, respectively.
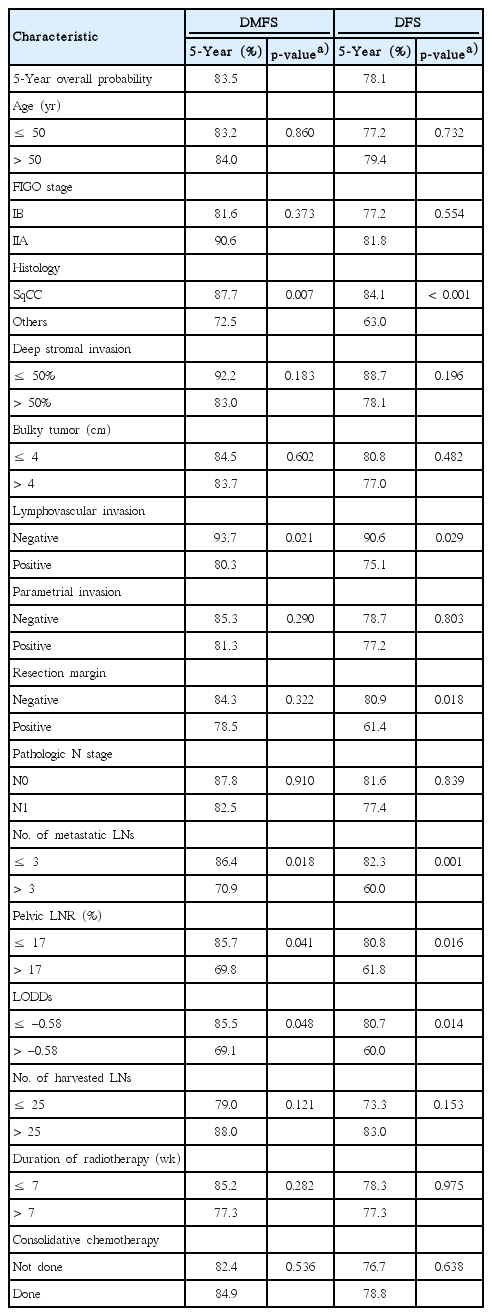
In univariate analysis, non-SqCC (p=0.007), the presence of LVI (p=0.021), mPLNs > 3 (p=0.018), pelvic LNR > 17% (p=0.041), and LODDs > ‒0.58 (p=0.048) were significant poor predictors for DMFS. However, no survival difference was observed according to the number of harvested LNs (p=0.121), duration of RT (p=0.282), or consolidative CTx (p=0.536). As for DFS, the survival rate was significantly different according to following factors: non-SqCC (p < 0.001), presence of LVI (p=0.029), mPLNs > 3 (p=0.001), pelvic LNR >17% (p=0.016), and LODDs > ‒0.58 (p=0.014), and RM positivity (p=0.018), respectively. DFS was not influenced by the number of harvested LNs (p=0.153), duration of RT (p=0.975), or consolidative CTx (p=0.638). These results are presented in Table 2.
Interestingly, pathologic N stage itself was independently associated with neither DMFS (p=0.910) nor DFS (p=0.839) (Fig. 1A). As the cutoff value of mPLN determined by maximal chi-square test was 3, we compared survival differences between three groups of patients: patients with pN0, patients with 1-3 mPLNs, and patients with mPLNs more than 3. Survivals of patients with 1-3 mPLNs were significantly higher (5-year DMFS, 86.0%; 5-year DFS, 82.7%) than those with > 3 mPLNs (5-year DMFS, 70.9%, p=0.017; 5-year DFS, 60.0%, p=0.001). However, survival differences between patients with pN0 and ≤ 3 mPLNs were not statistically significant (5-year DMFS, 87.7%, p=0.616; 5-year DFS, 81.6%, p=0.535). Fig. 1B depicts survival curves according to these three groups.
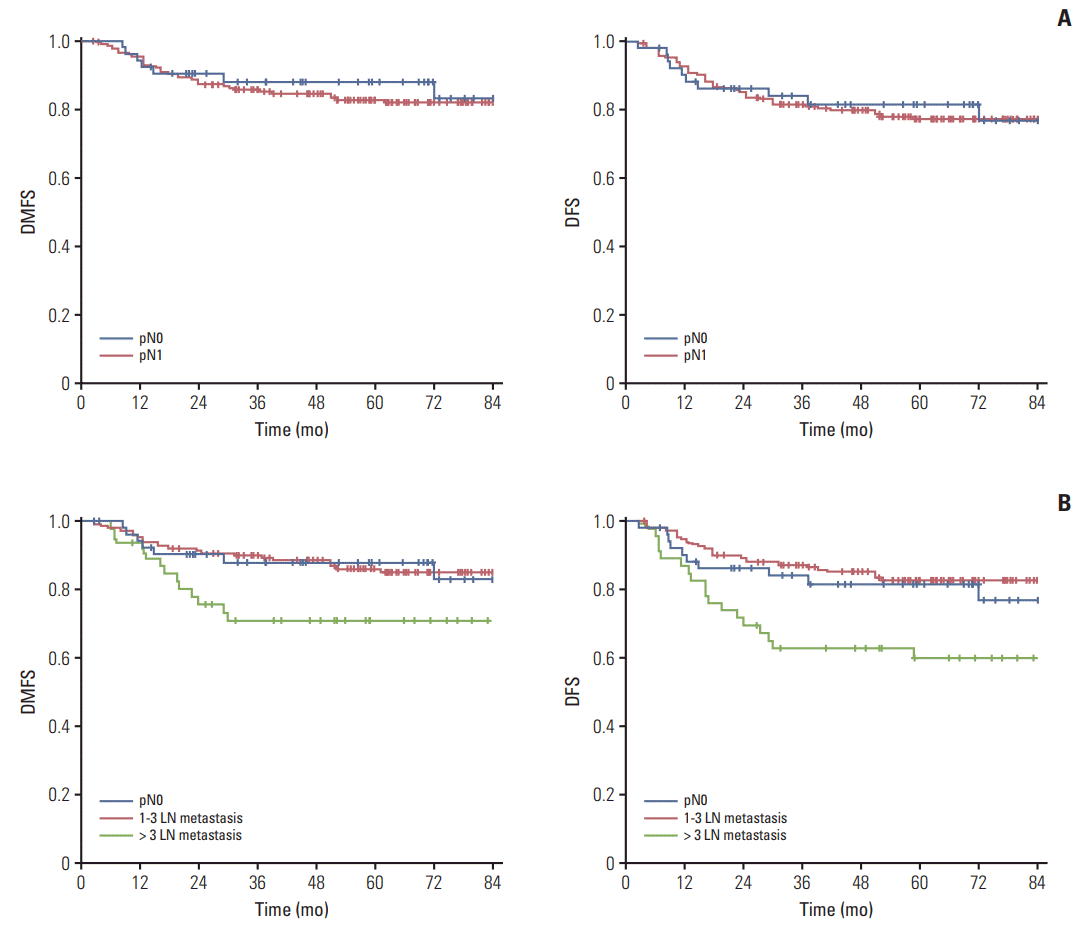
Distant metastasis-free survival (DMFS) and disease-free survival (DFS) curves according to pN stage (A), and number of metastatic pelvic lymph nodes (LNs) (B).
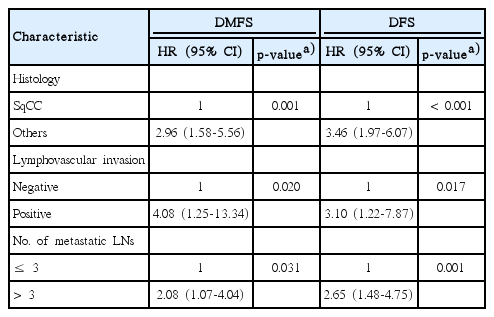
In multivariate analysis (Table 3), both DMFS and DFS were significantly affected by three factors: non-SqCC histology (DMFS: p=0.001; HR, 2.96 and DFS: p < 0.001; HR, 3.46), LVI (DMFS: p=0.020; HR, 4.08 and DFS: p=0.017; HR, 3.10), and mPLNs > 3 (DMFS: p=0.031; HR, 2.08 and DFS: p=0.001; HR, 2.65). S3 Fig. shows survival curves according to these factors. Other LN-related parameters (pN stage, LNR, or LODDs) lost their significance after adjusting interaction between variables.
3. A scoring system for prediction of DMFS and DFS
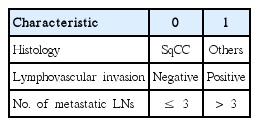
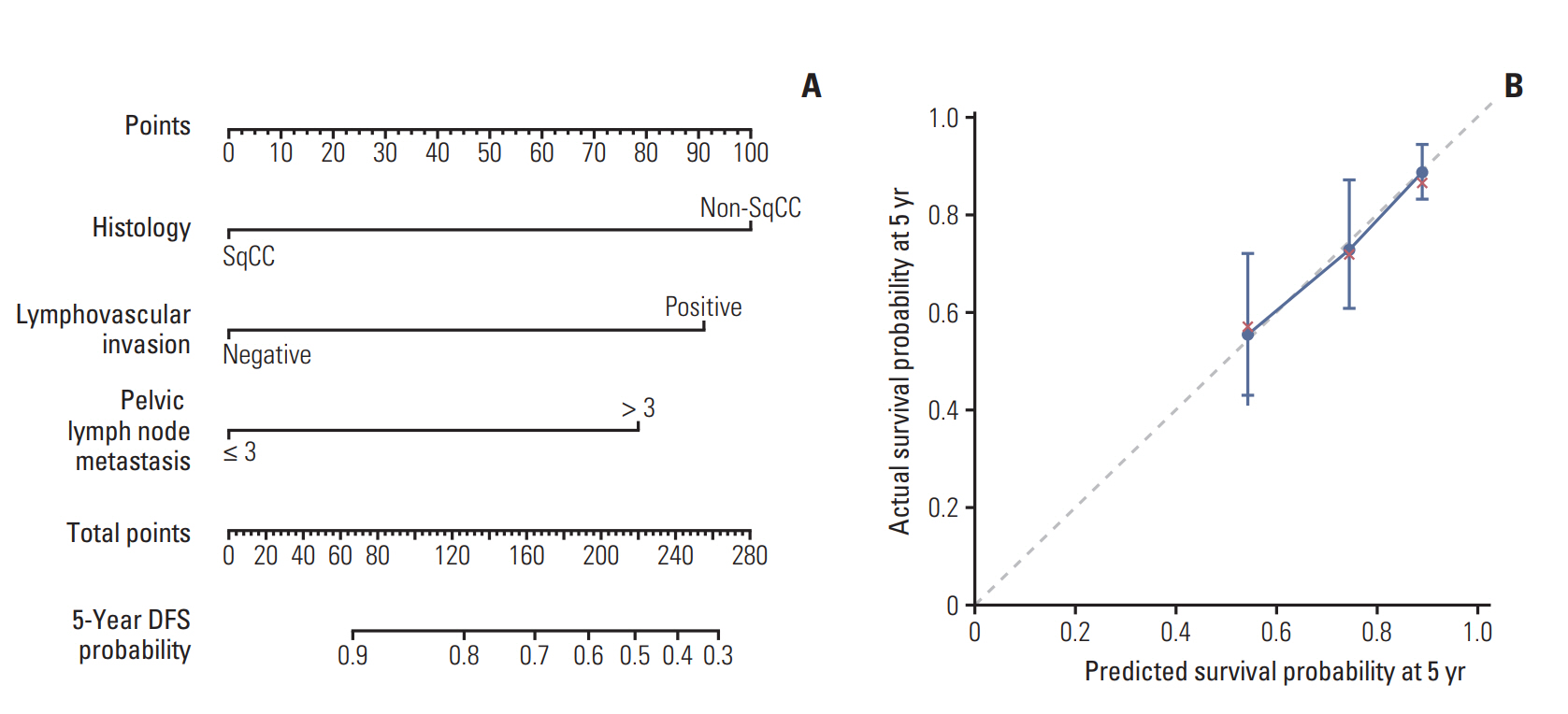
A scoring system was built based on the results of multivariate analysis (Table 4). Non-SqCC histology, presence of LVI, and mPLNs > 3 were counted independently as 1 point with total score ranging from 0 to 3. As a result, patients were stratified into four groups as follows: 28 patients (12.1%) with score 0, 125 patients (53.9%) with score 1, 69 patients (29.7%) with score 2, and 10 patients (4.3%) with score 3, respectively. The 5-year DMFS and DFS were 100% in patients with score 0. In contrast, 5-year DMFS and DFS were 50% and 40% in patients with score 3. In patients with score 1 and 2, 5-year DMFS and DFS were 88.6%/71.2% and 85.9%/62.1%, respectively (Fig. 2). The Harrell's C index of the scoring system for DMFS and DFS were 0.69 and 0.71.

Distant metastasis-free survival (DMFS) (A) and disease-free survival (DFS) (B) curves according to the scoring system.
Additionally, we developed prognostic nomogram for DFS using above three risk factors to specifically estimate individual patient’s prognosis (Fig. 3A, Supplementary Method). The nomogram suggested that non-SqCC histology had the largest impact on prognosis. The concordance index of our nomogram predicting 5-year survival probability was 0.69. The calibration plot depicted a good correspondence between the nomogram-predicted survival and actuarial survival rate at 5 years (Fig. 3B).
Discussion
The present study enrolled homogenous groups of patients diagnosed of FIGO IB-IIA cervical cancer without clinically involved LNs, treated by radical surgery and adjuvant CRT owing to postoperative high risk features. This study demonstrates that the number of mPLN shows best prognostic performance among parameters related to LN status. DMFS and DFS of patients with mPLN ≤ 3 did not differ from those with pN0. In contrast, the risk of recurrence in patients with mPLNs > 3 remained high even though postoperative CRT was performed. Additionally, we proposed a scoring system which can discriminate poor prognostic group of patients using three factors including tumor histology, LVI, and the number of mPLN. It is simple and correlates well with DMFS and DFS.
Some of well-known prognosticators such as bulky tumor size, deep stromal invasion, positive RM, and PM involvement for early cervical cancers did not affect survivals in the present study. As those factors are mainly derived from surgical series to establish indications of adjuvant treatment [8,15,16], proper prognostic factors in early cervical cancers with high risk features are currently unknown when standard treatment including radical surgery and postoperative CRT were performed. Our findings suggest that prognosticators for survivals in this specific setting would be different from those in surgical series and should be defined separately.
The LN status affects prognosis of patients and several parameters associated with LN status were suggested in the previous studies. It is agreed that pN1 disease is a heterogeneous group in terms of the location and the tumor burden of involved LN. Nodal staging system based on the number of metastatic LNs is widely used in other cancers of breast, stomach, and rectum [14]. In studies of patients with early stage cervical cancer treated with radical surgery followed by RT, some authors showed that the survival difference of patients between with one mPLN and ≥ 2 was statistically significant [7,15]. KROG1303 study showed that the hazard of death increases continuously with the number of mPLNs (up to 5) [11]. On the other hand, Tsai et al. [10] reported that patients with only one mPLN had achieved similar outcomes compared to those with pN0 (5-year DFS, 84% vs. 87%; p=0.48), and patients with ≥ 2 mPLNs had lower survival rates (87% vs. 61%, p < 0.001) than those with pN0. In the present study, there was no significant difference in survival between patients with pN0 and those with 1-3 mPLNs which is higher cut-off value than Tsai et al. [10]. The main difference between two is that whether concomitant CTx was administered or not. Possible explanation of the observed difference in the number of significant mPLN will be that the risk of recurrence, especially distant failure, might be attenuated by concomitant CTx in patients with 2-3 mPLNs.
The number of mPLNs directly depends on the extent of LN dissection. Inadequate LN dissection results in underestimation of LN metastasis. The prognostic advantage of LNR as a surrogate for LN status, which reflects the number of mPLN as well as the degree of LN dissection, has been recognized. Fleming et al. [17] reported decreased survivals in patients with high LNR (6.6% for progression-free survival, and 7.6% for OS) compared to those with low LNR in early stage cervix cancer. Li et al. [18] and Polterauer et al. [19] showed that the prognosis of patients with high LNR greater than 10% was poor. The present study suggests a cutoff value of 17% for LNR. As the median number of harvested LNs was 26 in the current study, which is not much different from the previous studies (Fleming et al. [17], median 19; Chen et al. [20], median 26), relatively high LNR cutoff in our study may be attributed to the improved survival of patients with pN1 in our study. The 5-year OS of pN1 patients in the present study was 84.9% which is higher than 22.4% (OS) in Chen et al. [20]. Additionally, Fleming et al. [17] reported a 5-year PFS of around 67% in patients with > LNR 6.6%, which is similar to those with LNR > 16% in our study (68.3%). More recently, Li et al. [18] and Zhou et al. [21] suggested cutoff values of LNR as 20% and 17% which is consistent with our results.
LODDs reflects not only the extent of LN dissection, but also negative LNs. It has the advantage of further discriminating patients with LNR close to zero or one [22]. For example, if the number of mPLN is zero, the LNR will be zero regardless of the dissection extent, but the LODDs will vary depending on the number of negative LNs (S4 Fig.). It has been studied actively in rectal and gastric cancers, but not in cervical cancers. Kwon et al. [12] reported the prognostic superiority of LODDs to the number of mPLNs and LNR in a patient group similar to our study, but it was limited due to small number of patients. In the present study, LODDs was a significant prognostic factor in univariate analysis, but lost its significance in multivariate analysis. In order for LODDs to acquire superior predictability, the number of negative LNs should be different among patients with mPLNs ≤ 3 with or without recurrences, but there was no significant difference (t test, p=0.458 for distant recurrence, p=0.2488 for overall recurrence).
In addition to the number of mPLN, our study revealed that LVI and histologic type were meaningful factors for predicting DFS as well as DMFS. The prognostic significance of LVI has been reported in many studies including GOG 49 [8] and is still an important prognosticator even after adjuvant RT [23]. In addition, LVI has been reported to be significantly associated with pelvic LN metastasis in stage IB cervical cancer [24]. Similarly, LVI was associated with mPLN (chisquare test, p=0.002) in our study, but it was an independent prognostic factor for both DMFS and DFS after adjusting interaction between two variables. These are in line with previous studies.
ADC in cervix cancer has been considered to have biological aggressiveness and to be more resistant to adjuvant treatment [25,26]. In the present study, ADC histology carried a poor prognosis with 71.8% of DMFS and 61.8% of DFS at 5 years compared to SqCC with 87.7% of DMFS and 84.1% of DFS at 5 years, respectively. The significantly lower survivals of ADC are consistent with the findings of Hosaka et al. [25] and Galic et al. [27]. In the study of Intaraphet et al. [28], the risk of cancer-related death was significantly higher in patients with small cell neuroendocrine carcinoma or ADC than those with SqCC. Of note, an unfavorable prognosis of ADC was observed only in advanced stages while there was no significant survival difference in early stages [28]. More recently, Winer et al. [29] also reported that equivalent survival outcomes regardless of histologic type were achieved in patients with early stage cervix cancer. The discrepancies between studies may be explained by the difference of patient characteristics: all patients in our study have early stages, but belong to the high-risk group. It could be supported by the study of Mabuchi et al. [30] showing that ADC was an independent poor prognostic factor for survivals in intermediate- and high-risk group, but not in low-risk group of patients with early cervical cancer.
Our study is limited inherently by its retrospective nature: the data were collected from 13 institutions in a retrospective manner. It resulted in the following weakness: data on the performance status of patients, pretreatment anemia, transfusion, and treatment-related toxicities were missing and unable to analyze. In addition, favorable treatment outcomes of our study may suggest a potential selection bias. However, to the best of our knowledge, the present study is the largest study focusing on early cervical cancer patients without clinical LN involvement carrying high-risk factors who underwent radical surgery followed by adjuvant CRT. For this specific patient population, our study suggested three prognostic factors including histologic type, LVI, and the number of mPLN for DMFS and DFS, and provides simple scoring system for predicting DMFS and DFS. It is worthwhile to identify patient groups at risk of treatment failure despite adjuvant CRT.
In conclusion, the risk of recurrence, especially distant failure, remains high when adjuvant CRT is performed in patients with mPLNs > 3. New prognostic scoring system consisted of mPLNs > 3, presence of LVI and non-SqCC is suggested, and it could stratify patients according to risk of recurrence in early cervix cancer with high-risk factors. Further treatment options such as consolidative CTx after adjuvant CRT should be considered to improve treatment outcomes in patients having poor prognostic factors and prospective trials are warranted.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.