Risk Factors for a False-Negative Result of Sentinel Node Biopsy in Patients with Clinically Node-Negative Breast Cancer
Article information
Abstract
Purpose
Although sentinel lymph node biopsy (SLNB) can accurately represent the axillary lymph node (ALN) status, the false-negative rate (FNR) of SLNB is the main concern in the patients who receive SLNB alone instead of ALN dissection (ALND).
Materials and Methods
We analyzed 1,886 patientswho underwent ALND after negative results of SLNB, retrospectively. A logistic regression analysis was used to identify risk factors associated with a false-negative (FN) result. Cox regression model was used to estimate the hazard ratio of factors affecting disease-free survival (DFS).
Results
Tumor located in the upper outer portion of the breast, lymphovascular invasion, suspicious node in imaging assessment and less than three sentinel lymph nodes (SLNs) were significant independent risk factors for FN in SLNB conferring an adjusted odds ratio of 2.10 (95% confidence interval [CI], 1.30 to 3.39), 2.69 (95% CI, 1.47 to 4.91), 2.59 (95% CI, 1.62 to 4.14), and 2.39 (95% CI, 1.45 to 3.95), respectively. The prognostic factors affecting DFS were tumor size larger than 2 cm (hazard ratio [HR], 1.86; 95% CI, 1.17 to 2.96) and FN of SLNB (HR, 2.51; 95% CI, 1.42 to 4.42) in SLN-negative group (FN and true-negative), but in ALN-positive group (FN and true-positive), FN of SLNB (HR, 0.64; 95% CI, 0.33 to 1.25) did not affect DFS.
Conclusion
In patients with risk factors for a FN such as suspicious node in imaging assessment, upper outer breast cancer, less than three harvested nodes, we need attention to find another metastatic focus in non-SLNs during the operation. It may contribute to provide an exact prognosis and optimizing adjuvant treatments.
Introduction
Nevertheless, molecular profiles are acknowledged promising prognostic factor, axillary lymph node (ALN) status is still the most important prognostic factor in breast cancer patients. This exact evaluation of ALN status is important. Sentinel lymph node biopsy (SLNB) represents the status of axillary involvement with more than 90% accuracy and develops a less complication than conventional axillary lymph node dissection (ALND) [1,2]. Larger randomized clinical trials have confirmed that the SLNB alone is a sufficient method in terms of disease-free survival (DFS), overall survival (OS), and moreover regional node recurrence event compared to ALND in sentinel lymph node (SLN)‒negative breast cancer patients [3,4]. The axillary recurrence after a tumor-negative SLNB on 14,959 patients showed that only 67 women developed an axillary recurrence, resulting in a recurrence rate of 0.3% [5]. Several randomized controlled trials also showed that axillary recurrence in SLN-negative patients was very low [1,3,4]. According to these results, SLN-negative patients do not undergo complete ALND anymore, but we still have the issue of false-negative (FN) in SLNB alone patients. False-negative rate (FNR) of the procedure was between 4.6% and 16.7% [6] with an average of 8.4% overall [7]. In case of FN, the remnant disease may potentially influence on poor clinical outcomes. ALN metastasis remain the most significant prognostic factor that dictates the use of adjuvant chemotherapy (CTx) [8,9]; however, in recent SLNB alone era a FN of SLNB can lead to understanding of disease and cannot provide exact information to make decisions for adjuvant treatment. The issue of FN has made the hesitation in the omission of further lymph node dissection in SLN-negative patients. Therefore, we need to pay particular attention to reducing FN in SLNB. The aim of the present study is to identify the risk factors for a FN of SLNB, and to analyze its clinical significance in patients with breast cancer.
Materials and Methods
1. Patients
We retrospectively analyzed the data of patients who were surgically treated for primary invasive breast cancer and who underwent a SLNB followed by completion of an ALND between January 1998 and December 2013 at Severance Hospital and Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Only patients whose SLNB results were negative for malignancy documented in the permanent section were enrolled in the present study. Women with carcinoma in situ or microinvasive carcinoma were excluded. We also excluded those who had received neoadjuvant CTx or preoperative hormonal therapy. The study was approved by the Institutional Review Board of Severance Hospital.
The clinical data from each patient was reviewed, and pathological findings, including tumor size, tumor grade, presence of multifocal or multicentric disease, and the number of lymph node metastases, were recorded. The results of estrogen receptor and progesterone receptor analysis were also recorded. The pathologic T stage and lymph node (N) stage were classified according to the seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control classification system [10]. The modified Scarf-Bloom-Richardson grading system was used for tumor grading. Most of the patients underwent breast and axillary ultrasound (US), breast magnetic resonance imaging (MRI) or positron emission tomography/computed tomography (PET-CT) for preoperative evaluation. Preoperative imaging studies were reviewed to evaluate axillary lymph node status. The suspicious finding of ALN metastasis based on the radiologist’s report, in any of these imaging studies was considered as positive image finding. Adjuvant systemic therapy and/or radiotherapy were considered according to the standard guideline based on patient age, primary tumor characteristics, and ALN status. Anti-hormone therapies were used for patients according to their hormone receptor status.
We categorized the results of SLNB as FN, true-negative (TN), and true-positive (TP). A FN SLN was defined as negative SLNs but tumor-positive in non-SLNs. TN SLN was defined as negative SLN and TP SLN was defined as positive SLN.
2. Procedure of SLNB
The SLNB was performed using technetium-99m (99mTc) tin colloid. Intradermal injection of 0.4 mL 30 MBq (0.8 mCi) 99mTc tin colloid diluted in normal saline solution was performed in 3-4 subareolar and intradermal areas. SLN were determined by employing a gamma detector in the operating room (Gamma Detection System, Neoprobe Corporation, Dublin, OH). The lymph node showing the highest radioactivity was dissected, after which the gamma detector was used again to confirm the correct lymph node. All radioactive lymph nodes with a count equal to or greater than 10% of the highest radioactive lymph node were removed. When suspicious lymph nodes were found after a SLNB that were not detected by the gamma probe, they were removed and examined as non-SLNs. All patients underwent ALND after a SLNB.
3. Statistical methods
A chi-square test was used to compare characteristics of the FN and TN groups. A logistic regression analysis was used to identify the risk factors for a FN in a SLNB. The Kaplan-Meier method and Cox regression model were used for the survival analysis. To control for differences in the baseline characteristics in each TP and FN group, a propensity score matching was used with match tolerance 0.001 and 1:1 ratio. The model included survival related characteristics, such as age at diagnosis, T stage, N stage, lymphovascular invasion, nuclear grade, human epidermal growth factor receptor 2 (HER2) status, anti-hormonal therapy, and type of breast surgery. We have incorporated changes in surgical technique by incorporating. All statistical analyses were performed using the SPSS ver. 24.0 (IBM Corp., Armonk, NY). A p-value less than 0.05 was considered to indicate a statistically significant difference.
Results
To assess risk factors for false-negativity in SLNB, 1,886 patients who underwent back up ALND after negative results of SLNB were enrolled in the present study. Among them, 1,707 patients have TN results, 179 patients were revealed to have a FN in the SLNB, and 24 patients had more than four positive axillary lymph nodes. The clinicopathologic characteristics of patients are presented in Table 1. Compared to those with a TN, factors found to be significantly associated with a FN in the SLNB included a primary tumor size > 2 cm, HER2-positive, lymphovascular invasion (LVI), positive results of preoperative axilla image studies and tumor location in the upper outer breast. There were patients with a single harvested SLN during the procedure. These represented 78 patients (43.6%) in the FN group and 520 patients (30.4%) in the TN group, respectively. There were 77 patients (45.8%) with suspicious imaging study result in the FN group and 372 patients (22.8%) in the TN group. Adjuvant CTx and radiation therapy were provided more frequently in those with a FN in the SLNB (p < 0.001 and p=0.033, respectively) (Table 1).

Clinicopathological characteristics between false-negative and true-negative group for sentinel lymph node biopsy
In the univariate analysis of risk factors for false-negativity in SLNB, odds ratio of tumor size > 2 cm, tumor in the upper outer quadrant of the breast, ductal histologic type, the presence of LVI, and positive results in imaging assessment were 1.70, 1.78, 1.88, 2.51, and 2.861, respectively. Three or more dissected SLNs had a statistically significant lower odds ratio for a FN in the SLNB than 1 or 2 dissected SLNs (odds ratio of 0.57 and 0.37, respectively). A multivariate logistic regression model identified that tumor located in the upper outer quadrant of the breast, the presence of LVI, positive result in imaging assessment, and the number of dissected SLNs (≤ 2) still remained significant independent risk factors for a FN in the SLNB (adjusted odds ratio of 2.10 [95% confidence interval, 1.30 to 3.39], 2.69 [95% confidence interval, 1.47 to 4.91], 2.59 [95% confidence interval, 1.62 to 4.14], and 2.39 [95% confidence interval, 1.45 to 3.95], respectively) (Table 2).
During the median 62.0 months (range, 1 to 174 months) of follow-up period, 18 patients (10.1%) had a recurrence and nine patients (5.0%) died in the FN group (n=174). Two patients (1.1%) had a loco-regional recurrence, and 16 (8.9%) had a systemic relapse. In the TN group (n=1,707), 72 (4.2%) patients had a recurrence, and 53 (3.1%) women died. Twenty-two of 72 (1.3%) had a loco-regional recurrence, and 50 (2.9%) had a systemic relapse. We also analyzed the survival of TP patients to see whether there exists a difference between FN and TP among ALN-positive patients according to SLN positivity. In TP group, 10.2% patients had experienced recurrence and 5.3% of them died. Among them, 2.3% had loco-regional recurrence and 7.9% had systemic recurrence.
In multivariate analysis, the prognostic factors for DFS in SLN-negative group (TN and FN) were tumor size > 2 cm (hazard ratio [HR], 1.86; p=0.009) and false-negativity (HR, 2.51; p=0.002). On the other hand, in the ALN-positive group (TP and FN), false-negativity (HR, 0.64; p=0.191) did not affect the prognosis of the DFS and only tumor size and nodal status were statistically significant prognostic factors (Table 3). The 5-year DFS rate and OS rate in the FN group were 89.2% and 94.6%, respectively, compared to 95.5% and 97.3% in the TN group (Fig. 1). To compensate for differences of factors affecting survival between FN and TP group, patients were selected in each group and baseline characteristics of each patient were well balanced (S1 Table). The adjusted 5-year DFS rate was same in both groups (89.3%). OS rate was 96.1% in the FN group and 96.7% in the TP group. There was no statistical difference between FN and TP in terms of survival outcomes (S2A and S2B Fig.).
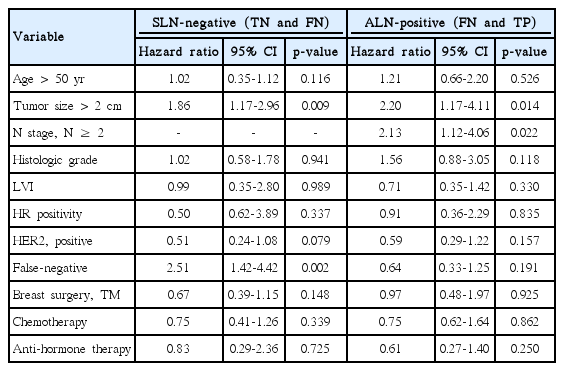
Multivariate analysis of prognostic factor for disease-free survival in patients undergoing sentinel lymph node biopsy
Discussion
ALN status is still one of the most important prognostic factors, and provide important information to decide an appropriate adjuvant treatment in breast cancer patients. SLNB has become the standard treatment of choice for evaluation of ALN status after releasing the results of NSABP B-32 trial [4]. The majority of surgeons does not complete ALND in patients with SLN-negative. In the NSABP B-32 trial, there was 9.8% FNR in SLNB followed by ALND group [11], considering that, there might be about 10% FN patients in SLNB alone group. Although, there was no significant difference in survival outcomes between two groups [4], about 10% of hidden FN patients could not obtain accurate information of ALN status compared with TN patients and were spared from appropriate adjuvant treatments. In recent SLNB alone era, information about ALN status is acquired through SLNB only. Therefore, efforts should be made to decrease the FN in SLNB.
There are several studies that have identified clinical factors predictive of a FN in a SLNB [12-17]. They mentioned tumor size, multifocal-multicentric disease, location of tumor, and number of removed SLN as risk factors. All of these studies were focused on FNR, in which the FN results were compared with TP results. In clinical practice, however, distinguishing whether a negative result in a SLNB is truly negative or actually positive is more important. In the present study, we have assessed the risk factors of FN in SLNBan d analyzed effect of false-negative results to patients’ survival. Tumor location, LVI, positive result in imaging assessment, and the number of dissected SLNs were the independent factors associated with a FN in the SLNB.
Among these risk factors, we newly found that patients with suspicious nodes in any kinds of preoperative imaging studies had a greater risk of FN with odd ratio 2.59 in a SLNB. The sensitivity and specificity to detect ALN metastasis with preoperative axillary US in the literature ranges from 25% to 87% and 77% to 100%, respectively [18-20]. Combining axillary US with breast MRI or PET-CT may enhance in predicting ALN metastasis because multimodal imaging studies improve the diagnostic performance compared with axillary US alone [21-23]. Nevertheless, preoperative suspicious finding in imaging studies is not enough to determine ALND without SLNB. Patients with suspicious ALN in imaging modality who showed negative result from preoperative fine needle aspiration or core needle biopsy could be indicated for SLNB if they do not have matted ALN. A possible reason for increased FNR in suspicious ALNs in imaging studies is that SLNs and radiologically suspicious nodes may not be identical. Wang et al. [24] reported that 38.3% of suspicious ALNs in US were indeed SLNs and FNR was decreased from 11.3% to 2.8% when SLNB with additional US-guided suspicious node excision was done. Generally, the metastatic tumor burden in suspicious lymph nodes in imaging studies is larger than that of benign looking nodes. The increased tumor burden may obstruct the perinodal lymphatic channel; then lymphatic drainage might bypass the true metastatic ALN which can lead to FN of SLNB. In the present study, only 17.1% of patients with suspicious node in imaging studies had metastasis, which suggest that preoperative radiological suspicious ALNs are not contraindication for SLNB. However, clinicians should be more careful in evaluating SLN and consider a potential of FN during a SLNB in such cases.
Although there are few studies that report LVI as a risk factor for a FN in a SLNB, we found that patients with LVI had FN more frequently. Lymphatic channels that became progressively infiltrated by tumor cells may not allow the passage of radioactive dye [25]. This might explain the positive correlation of LVI with FN in SLNB. Several studies have already shown that LVI is associated with increased risk of axillary involvement [26,27]. Consequently, a tumor with LVI may have a greater chance of a FN in a SLNB by lymphatic obliterans.
Other risk factors such as tumor location [12,13] and the number of dissected SLNs [12,15,17] were also shown to be significant risk factors for a FN in other studies. Our findings are concordant with previous studies that FN results are more likely to be observed when a tumor is located in the upper outer quadrant of the breast and when only a single SLN is harvested. The correlation between the number of removed SLN and FNR has been reported in previous studies. However, the best cut-off value of number of harvested SLN of reducing the FNR is inconsistent. Some studies suggested up to four removed SLNs increased diagnostic accuracy [28]. Our result shows less than three harvested SLNs increase the FN with 2.3 of odds ratios.
The Early Breast Cancer Trialists’ Collaborative Group showed that CTx is important for decreasing recurrence and increasing survival in early breast cancer patients [29]. In clinical practice, ALN metastases have been recognized as an essential indication for adjuvant CTx. In terms of optimizing adjuvant treatments, the risk of FN can be more importantly considered in individual patients. FN in SLNB may deprive the patients who actually have positive axillary lymph nodes of the opportunity for CTx, and adversely influence survival outcomes. In the present study, we have analyzed the clinical significance of FN group compared to TN group. False-negative result of SLNB is most powerful independent prognostic factor (HR, 2.51; 95% confidence interval, 1.42 to 4.42) affecting DFS in this group, but this effect was not showed in FN and TP group. Patients in the FN group presented a similar loco-regional recurrence rate compared to patients in the TN group (1.1% vs. 1.5%), but the FN group had a greater systemic recurrence rate than the TN group (8.9% vs. 2.9%, p < 0.001, data not shown). Patients with FN in the SLNB had a worse DFS than those with TN in the SLNB, despite 96.6% of patients with a FN in the SLNB receiving adjuvant CTx. Moreover, some patients had extended axillary disease, and 13.4% of women were revealed to have four or more positive ALNs. The National Comprehensive Cancer Network clinical practice guidelines advocate that irradiation to the infraclavicular region and supraclavicular area should be recommended for patients with four or more positive ALNs [9]. With an understanding that these patients might result in undertreatment, we can expect that they would probably display a poorer prognosis. Thus, we suggest that making an effort to discover metastatic non-SLNs is necessary in patients with a high risk for a FN in a SLNB. It may reduce the chance of masking the FN cases and allow more appropriate adjuvant treatment including CTx and regional radiotherapy for these patients. Refined adjuvant treatments by identifying FNs may contribute to ameliorate oncologic outcome. Preoperative multi-imaging studies can provide information on risk factors for a FN, although further study is needed to evaluate the appropriate combination of imaging modalities. We also compared the survival of FN and TP patients to see whether there exists a difference according to SLN positivity which resulted in no difference between two groups as expected since these patients all received ALND.
The present study has several limitations. Our data were based on a retrospective cohort from two different institutes. Protocol of pathologic evaluation for SLNs was not identically established in an aspect of serial section or additional immunohistochemical stain which can influence FN of SLNB. However, the pathologic evaluation protocol has been standardized in the later period of this study and we analyzed a relatively large number of patients compared to previous studies. Another limitation is that we did not confirm all of radiologically suspicious ALNs with core needle biopsy or fine needle aspiration cytology.
In summary, SLNB has been accepted standard axillary staging surgery for patients with clinically node-negative breast cancer. We found risk factors for a FN of a SLNB and showed a worse outcome of patients with FN compared with those with TN. In patients with LVI, tumor located at the upper outer quadrant of the breast, positive ALN in preoperative imaging assessment, and with less than three harvested SLNs, additional effort to find suspicious non-SLNs during SLNB may contribute to optimizing adjuvant treatments.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.

