The Prognostic Value of Treatment-Related Lymphopenia in Nasopharyngeal Carcinoma Patients
Article information
Abstract
Purpose
This study was conducted to evaluate the prognostic value of treatment-related lymphopenia in patients with nasopharyngeal carcinoma (NPC).
Materials and Methods
A total of 413 consecutive stage II-IVb NPC patients treated with concurrent chemoradiotherapy (CCRT) were enrolled. The overall survival (OS), progression-free survival (PFS), and distant metastasis-free survival (DMFS) were calculated with the Kaplan-Meier method, and differences were compared using the log-rank test.
Results
A minimum (mini)–absolute lymphocyte counts (ALC) of < 390 cells/μL or ALC after 3 months of CCRT (post3m-ALC) < 705 cells/μL was significantly associated with worse outcome than mini-ALC ≥ 390 cells/μL (OS, p=0.002; PFS, p=0.005; DMFS, p=0.004) or post3m-ALC ≥ 705 cells/μL (OS, p < 0.001; PFS, p < 0.001; DMFS, p=0.001). Patients with lymphopenia (mini-ALC < 390 cells/μL and post3m-ALC < 705 cells/μL) had a worse prognosis than those without lymphopenia (mini-ALC ≥ 390 cells/μL and post3m-ALC ≥ 705 cells/μL) (OS, p < 0.001; PFS, p < 0.001; DMFS, p < 0.001). Multivariate analysis revealed that post3m-ALC was an independent prognostic factor for OS (hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.12 to 2.78; p=0.015), PFS (HR, 1.86; 95% CI, 1.23 to 2.82; p=0.003), and DMFS (HR, 1.87; 95% CI, 1.13 to 3.08; p=0.014). Multivariate analysis also revealed that patients with lymphopenia had a high risk of death (HR, 3.79; 95% CI, 1.75 to 8.19; p=0.001), disease progression (HR, 2.93; 95% CI, 1.59 to 5.41; p=0.001), and distant metastasis (HR, 3.89; 95% CI, 1.67 to 9.10; p=0.002). Multivariate analysis performed with time dependent Cox regression demonstrated ALC was an independent prognostic factor for OS (HR, 0.995; 95% CI, 0.991 to 0.999; p=0.025) and PFS (HR, 0.993; 95% CI, 0.988 to 0.998; p=0.006).
Conclusion
Treatment-related lymphopenia was a poor prognostic factor in NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC), which is the most common malignancy arising from the nasopharynx epithelium, is especially prevalent in southern China. Radiation therapy (RT) is the primary treatment for NPC [1,2]. Specifically, the use of concurrent chemotherapy and intensity-modulated radiation therapy (IMRT) has been shown to provide a survival benefit [3-5], but 20%-30% of patients still die from tumor relapse [6]. Currently, the TNM stage is the mainstay measurement method used to predict the prognosis of NPC patients. Nevertheless, outcomes vary among patients within the same staging category and histological classifications because of the heterogeneity of the tumor [7,8]. Accordingly, methods for investigating efficient prognostic factors and stratifying patients with a high risk of tumor relapse need to be developed.
The immune system is believed to be important in the prevention of cancer development and progression. Specifically, lymphocytes, which are an essential component of host immunity, play a critical role in the destruction of residual tumor cells and related micrometastases [9,10]. RT is known to induce immunosuppression, regardless of the administration of chemotherapy, and to directly suppress immune function by destroying mature circulating lymphocytes [11-14]. In our daily clinical work, we have observed decreases in the lymphocyte population over the course of RT, and this population subsequently recovers in NPC patients. Moreover, previous studies of other cancer types demonstrated that lymphopenia was associated with poor patient prognosis [15-19]. Therefore, we analyzed the ability of the decline in absolute lymphocyte counts (ALC) during concurrent chemoradiotherapy (CCRT) and the latter phase after the completion of CCRT to predict clinical outcome. We hypothesize that treatment-related lymphopenia is correlated with poor patient prognosis and may provide an additional dimension for risk stratification and individualized therapy.
Materials and Methods
1. Patients
A total of 427 previously untreated NPC patients were enrolled between February 2007 and December 2012. The eligibility criteria were as follows: (1) biopsy-proven World Health Organization 2- or 3-histopathologic type NPC; (2) stage II-IVb disease according to the seventh edition of the International Union against Cancer/American Joint Committee on Cancer staging system; (3) no evidence of distant metastases; (4) Eastern Cooperative Oncology Group performance status grade 0 or 1; (5) aged 18 years or older; (6) absence of secondary malignancy, pregnancy or lactation; and (7) adequate hematological function (white blood cell counts ≥ 4,000/μL and platelet counts ≥ 100,000/μL), adequate renal function (creatinine clearance ≥ 50 mL/min), and adequate hepatic function (serum bilirubin level < 1.5 mg/dL). Fourteen patients (eight patients who were lost during follow-up, one with heart deficiency, three with liver deficiency, and two pregnant patients) were excluded from the study, giving a final cohort of 413 patients.
2. Pretreatment evaluation
Pretreatment assessment consisted of a medical history, complete physical examination, fiber-optic nasopharyngoscopy, magnetic resonance imaging (MRI) of the nasopharynx and neck, electrocardiography, chest radiography, abdominal sonography, bone scan or whole-body fluorodeoxyglucose positron emission tomography/computed tomography, as well as complete blood count, renal and liver function tests. Epstein-Barr virus (EBV) serology was assessed using a previously described immunoenzymatic assay [20], and the plasma level of EBV DNA was measured by real-time quantitative polymerase chain reaction [21,22]. All patients provided written informed consent for enrolling in our prospective database and the study was approved by the Clinical Research Committee of the study institute.
3. Treatment
All patients received 2-3 cycles of concurrent cisplatin (80-100 mg/m2) as an intravenous infusion every 21 days. IMRT was administered to all identified patients in the study. The IMRT plan was designed based on previous studies [6,23] (Supplementary Methods). All patients were treated according to the treatment principles for NPC patients at our institute.
4. Lymphocyte counts examination
The complete blood count (CBC) was determined using a Sysmex XE-5000 automated hematology analyzer (Sysmex, Kobe, Japan). The ALC were assessed prior to CCRT and weekly thereafter until completion of the CCRT. The ALC was also collected three months after CCRT. Additional CBC tests were performed for patients who developed a specific condition during treatment. The ALC before CCRT (pre-ALC), minimum ALC during CCRT (mini-ALC), ALC after the completion of CCRT (post-ALC), and ALC 3 months after CCRT (post3m-ALC) were evaluated.
5. Outcome and follow-up
Our primary study endpoint was the overall survival (OS), which was calculated from the first day of treatment until the day of death from any cause or patient censoring at the recent follow-up. The secondary endpoints included progression-free survival (PFS), which was calculated from the first day of treatment until the day of first tumor relapse, death from any course or patient censoring at the recent follow-up, and distant metastasis-free survival (DMFS), defined as the duration from the first day of treatment to the date of distant relapse or patient censoring on the most recent follow-up. After completion of treatment, the patients were examined every 3 months during the first 3 years and every 6 months thereafter until death. The patient history was obtained, and a physical examination and nasopharyngoscopy were performed at each follow-up visit. MRI of the nasopharynx and neck, chest X-ray imaging, abdominal sonography, and plasma EBV DNA measurements were routinely performed on an annual basis or upon a clinical indication of tumor relapse.
6. Statistical analysis
Categorical variables were assessed using Fisher exact test and the chi-square test, whereas continuous variables were analyzed with the t test. The cut-off values for the pre-ALC, mini-ALC, post-ALC, and post3m-ALC demonstrated maximum sensitivity and specificity for survival based on receiver operating characteristic curves of OS. Survival was analyzed using the Kaplan-Meier method and log-rank test. The hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model, and univariate and multivariate analyses were performed. Time dependent Cox regression modeling was also conducted. The data were managed and analyzed using the SPSS ver. 20.0 statistical software package (IBM Corp., Armonk, NY). Two-tailed p-values of < 0.05 were considered to indicate significant differences.
Results
The characteristics of the 413 patients are presented in Table 1. Within the median follow-up duration of 60 months (range, 6 to 112 months), 99 patients died, including 62 who died due to distant metastases, 23 who died due to local and/or regional relapse, six who died due to severe adverse events, three who died due to non-cancer causes, two who died due to secondary malignant tumors after treatment and three who died due to unknown causes. Furthermore, 82 patients developed distant metastases, and 34 exhibited local or regional relapse. The 5-year survival rates for all patients were as follows: OS, 81.6%; PFS, 75.1%; DMFS, 82.7%.
1. ALC over time
Among the 413 patients, the median pre-ALC was 1,800 cells/μL (range, 300 to 4,710 cells/μL), the median mini-ALC was 300 cells/μL (range, 80 to 1,400 cells/μL), the median post-ALC was 300 cells/μL (range, 80 to 3,000 cells/μL), and the median post3m-ALC was 970 cells/μL (range, 280 to 3,600 cells/μL) (Table 1). According to both the summary data from all patients and the patient subgroup data, the ALC exhibited a downward trend during RT until reaching the minimum value, then recovered (Fig. 1).
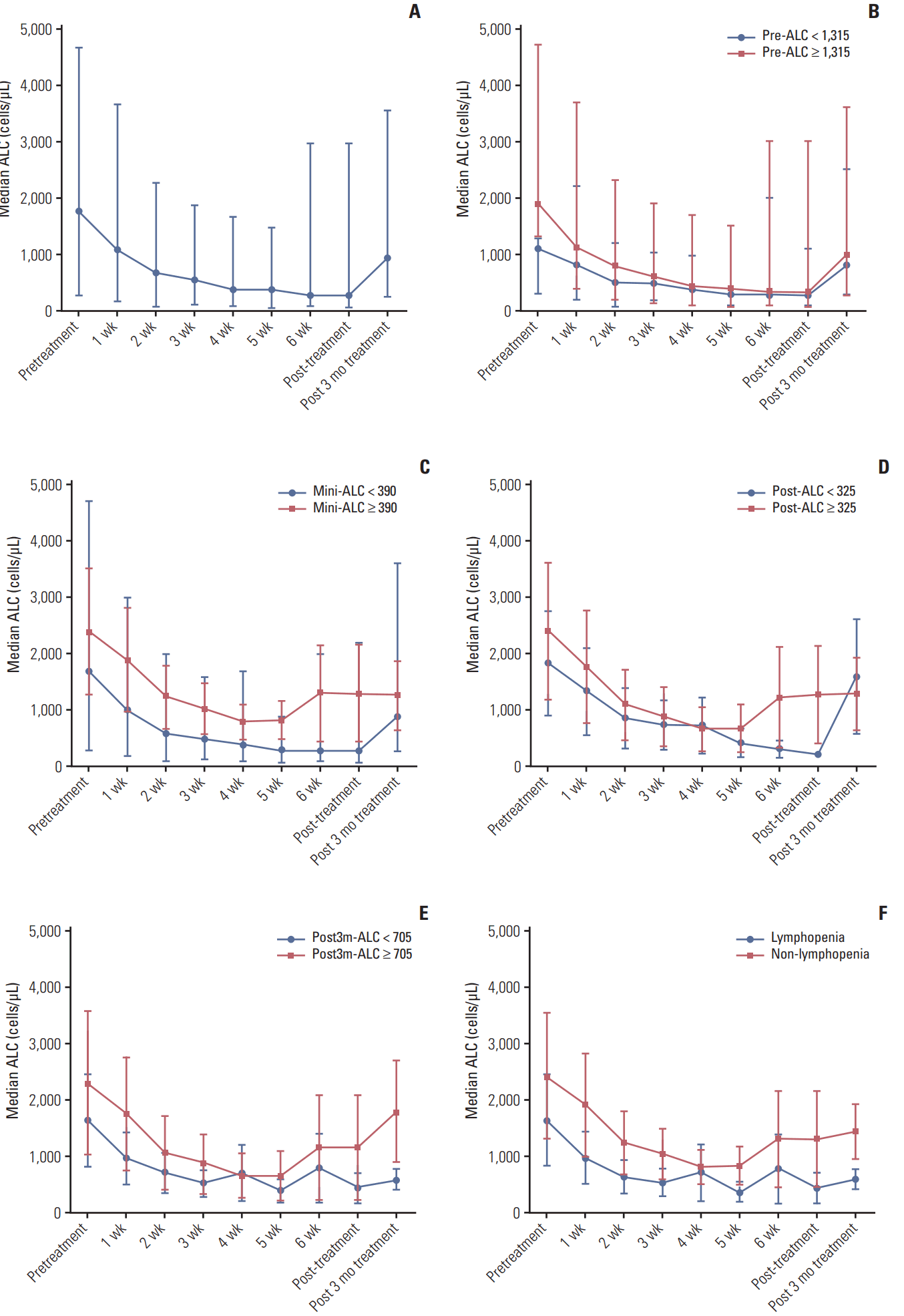
Variation in median ALC among all patients (A) and subgroups of patients stratified by the pre-ALC (< 1,315 cells/μL vs. ≥ 1,315 cells/μL) (B), mini-ALC (< 390 cells/μL vs. ≥ 390 cells/μL) (C), post-ALC (< 325 cells/μL vs. ≥ 325 cells/μL) (D), post3m-ALC (< 705 cells/μL vs. ≥ 705 cells/μL) (E), and mini-ALC combined with post3m-ALC (lymphopenia vs. nonlymphopenia) (F). ALC, absolute lymphocyte counts; pre-ALC, absolute lymphocyte counts before concurrent chemoradiotherapy; mini-ALC, minimum absolute lymphocyte counts during treatment; post-ALC, absolute lymphocyte counts after completion of treatment; post3m-ALC, absolute lymphocyte counts 3 months after completion of treatment.
2. Relationship between the variation in ALC and outcome
The cut-off values for the pre-ALC, mini-ALC, post-ALC, and post3m-ALC based on receiver operating characteristic curves were 1,315 cells/μL, 390 cells/μL, 325 cells/μL, and 705 cells/μL, respectively.
The OS differed significantly between the mini-ALC, post-ALC, and post3m-ALC groups. However, the OS did not differ significantly in the pre-ALC group (Table 2).
According to the survival curves, the PFS also differed significantly between the mini-ALC and post3m-ALC groups. Conversely, survival did not differ significantly between the pre-ALC and post-ALC groups (Table 2).
In the pre-ALC group, the DMFS differed between patients in the pre-ALC group, but this difference was not significant, whereas the differences among the mini-ALC, post-ALC, and post3m-ALC groups were all significant (Table 2).
The 5-year OS, PFS, and DMFS values of patients with a mini-ALC < 390 cells/μL or a post3m-ALC < 705 cells/μL were all significantly worse than those of patients with a mini-ALC ≥ 390 cells/μL or a post3m-ALC ≥ 705 cells/μL. Therefore, we defined patients with a mini-ALC < 390 cells/μL and post3m-ALC < 705 cells/μL as the lymphopenia group (n=86). Moreover, patients with a mini-ALC ≥ 390 cells/μL and post3m-ALC ≥ 705 cells/μL were defined as the non-lymphopenia group (n=95) (Table 3). Survival rates of patients in the lymphopenia group were worse than those of patients in the non-lymphopenia group (OS, p < 0.001; PFS, p < 0.001; DMFS, p < 0.001) (Table 2, Fig. 2).

Comparison of patients in the lymphopenia group (mini-ALC < 390 cells/μL and post3m-ALC < 705 cells/μL) with patients in the non-lymphopenia group (mini-ALC ≥ 390 cells/μL or post3m-ALC ≥ 705 cells/μL) in terms of overall survival (A), progression-free survival (B), and distant metastasis-free survival (C). mini-ALC, minimum absolute lymphocyte counts during treatment; post3m-ALC, absolute lymphocyte counts 3 months after completion of treatment.
Univariate analysis of all 413 patients demonstrated that N stage (p=0.001), the pretreatment EBV DNA level, the mini-ALC, the post-ALC and the post3m-ALC were significantly associated with OS (S1 Table). Additionally, N stage, the pretreatment EBV DNA level, the mini-ALC, and the post3m-ALC were significantly associated with PFS (S1 Table), and N stage, the pretreatment EBV DNA level, the mini-ALC, the post-ALC and the post3m-ALC were significantly correlated with DMFS (S1 Table). When ALC was re-analyzed as a time dependent variable, univariate analysis also demonstrated ALC was significantly correlated with OS, PFS and DMFS (S1 Table).
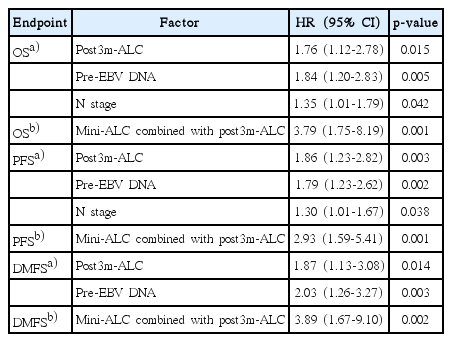
Multivariate analyses of all 413 patients were performed to further adjust for age, sex, T stage, N stage, family history, smoking, pretreatment EBV DNA level, VCA-IgA, EA-IgA, chemotherapy cycles, treatment days, dose of nasopharynx, dose of neck, pre-ALC, mini-ALC, post-ALC, and post3m-ALC. These analyses revealed that N stage (HR, 1.35; 95% CI, 1.01 to 1.79; p=0.042), the pretreatment EBV DNA level (HR, 1.84; 95% CI, 1.20 to 2.83; p=0.005) and the post3m-ALC (HR, 1.76; 95% CI, 1.12 to 2.78; p=0.015) were independent prognostic factors for OS (Table 4). Additionally, N stage (HR, 1.30; 95% CI, 1.01 to 1.67; p=0.038), the pretreatment EBV DNA level (HR, 1.79; 95% CI, 1.23 to 2.62; p=0.002), and the post3m-ALC (HR, 1.86; 95% CI, 1.23 to 2.82; p=0.003) were independent prognostic factors for PFS (Table 4), and the pretreatment EBV DNA level (HR, 2.03; 95% CI, 1.26 to 3.27; p=0.003) and the post3m-ALC (HR, 1.87; 95% CI, 1.13 to 3.08; p=0.014) were independent prognostic factors for DMFS (Table 4). When ALC was re-analyzed as a time dependent variable, multivariate analysis demonstrated it was an independent prognostic factor of OS (HR, 0.995; 95% CI, 0.991 to 0.999; p=0.025) and PFS (HR, 0.993; 95% CI, 0.988 to 0.998; p=0.006) (S2 Table).
For the 181 patients in the lymphopenia and non-lymphopenia groups, univariate analyses showed that patients in the lymphopenia group were at a higher risk of death (p < 0.001), disease progression (p < 0.001), and distant metastasis (p < 0.001) than those in the non-lymphopenia group (Table 4). We subjected data from the 181 patients to another multivariate analysis, which revealed that the ALC (lymphopenia vs. nonlymphopenia) was the only independent prognostic factor for OS (HR, 3.79; 95% CI, 1.75 to 8.19; p=0.001), PFS (HR, 2.93; 95% CI, 1.59 to 5.41; p=0.001), and DMFS (HR, 3.89; 95% CI, 1.67 to 9.10; p=0.002) (Table 4).
Discussion
The data presented in this study demonstrated that a decreased ALC during RT and after the completion of treatment is associated with poor prognosis among NPC patients. This decrease in the ALC was attributed to the immunosuppression induced by RT [11]. Previous studies demonstrated that RT directly destroys mature circulating lymphocytes, which exhibit significant DNA fragmentation, even at low radiation doses (< 1 Gy) [13,14]. The ability of radiation to decrease the circulating ALC has been well established in previous studies of other cancer types [12,24]. For example, Tang et al. [25] found that RT reduced lymphocyte counts independent of concurrent chemotherapy use. This decrease was most pronounced near the completion of RT, and lower lymphocyte minima during definitive RT were associated with worse patient outcomes [25]. Moreover, Grossman et al. [19] collected serial lymphocyte counts, prognostic factors, treatment data, and survival data from four independent solid tumor studies. Their study demonstrated that treatment-related lymphopenia increased the risk of death in each cancer cohort [19]. Furthermore, Cho et al. [26] investigated the mini-ALC during RT in NPC and showed that this parameter may predict a poor 5-year disease-specific survival. However, it should be noted that the sample size of their study was relatively small; therefore, we conducted this study of 413 patients to further validate the results and improve statistical outcomes. The ALCs before CCRT, during CCRT, after the completion of CCRT and 3 months after the completion of RT were all documented. According to our study, among several variables associated with treatmentrelated lymphopenia, the mini-ALC and post3m-ALC were strongly correlated with survival. Specifically, a mini-ALC < 390 cells/μL indicates an early 2-fold increase in the risk of early death. Furthermore, patients with a post3m-ALC < 705 cells/μL were at an even higher risk of death, disease progression and distant metastasis. When we combined these two ALC measurement time points, we found that lymphopenia (mini-ALC < 390 cells/μL and post3m-ALC < 705 cells/μL) was strongly correlated with a poor clinical outcome, specifically, a nearly 4-fold increase in the risk of early death and distant metastasis and a nearly 3-fold increase in the risk of disease progression. We assumed that the reduction of individual productivity of lymphocytes in response to RT and the extent of the tumor might both be relevant to lymphopenia. Based on this assumption, cancer progression might lead lymphocyte infiltration from peripheral blood into tumors and adjacent tissues, resulting in lymphopenia [26]. Thus, the reduction in the tumor volume due to RT may reduce the number of tumor infiltrating lymphocytes (TIL) and consequently increase the number of circulating lymphocytes. The degree to which the ALC declines and recovers may reflect the response to treatment. In most hospitals, a CBC is routinely obtained during treatment for NPC, and ALC data are easily obtained in a timely manner for rapid clinical implementation. Therefore, variations in the ALC could help us identify subpopulations sensitive to treatment and allow risk stratification for individualized therapy. Patients with lymphopenia experienced most of the treatment failures and therefore required intense treatment in subsequent phases, such as the administration of an additional target agent [27] or the inclusion adjuvant chemotherapy [5,28]. Moreover, additional immunotherapy could be administered to patients with treatment-related lymphopenia. For example, immunotherapy with EBV-specific cytotoxic T cells has been shown to be effective in post-transplant lymphoproliferative disorders [29], and the adoptive transfer of latent membrane protein 2–specific cytotoxic T lymphocytes has shown clinical efficacy in heavily pretreated patients with NPC [30,31]. We are initiating a phase II study of adoptively transferred TIL immunotherapy following CCRT in patients who are at a high risk of treatment failure [32]. Adoptive immunotherapy is a potential avenue for patients with treatment-related lymphopenia.
It should be noted that our study was subject to several limitations. Specifically, our data were unable to stratify the correlation between lymphocyte counts during or after RT and survival by lymphocyte type. Additionally, all patients in our study received CCRT, and the effects of radiotherapy and chemotherapy on lymphopenia could not be separately investigated. Finally, our data were exclusively obtained from one center; therefore, these results must be validated by other datasets.
In conclusion, our study found that treatment-related lymphopenia was correlated with poor prognosis in NPC patients. Therefore, lymphocytes should be considered a prognostic marker that may reflect the immunological status of patients during and after CCRT and stratify patients who are at a high risk of treatment failure. Future clinical trials and laboratory investigations to elucidate the immunology underlying treatment-related lymphopenia and the prevention of lymphopenia or restoration of immune function in NPC are warranted.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81425018, No. 81072- 226, No. 81201629, and No. 8160100709), the 863 Project (No. 2012AA02A501), the National Key Basic Research Program of China (No. 2013CB910304), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the SCI-Tech Project Foundation of Guangdong Province (No. 2014A020- 212103, No. 2011B080701034, No. 2011B031800161), the Health & Medical Collaborative Innovation Project of Guangzhou City (No. 201400000001), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2014BAI09B10), the PhD Start-up Fund of the Natural Science Foundation of Guangdong Province, China (No. 2016A- 030310221), the Cultivation Foundation for Junior Teachers in Sun Yat Sen University (No. 16ykpy28), the Sun Yat-Sen University Clinical Research 5010 Program, the Sun Yat-Sen University Cancer Center Clinical Research 308 Program, and the Fundamental Research Funds for the Central Universities.