A Locally Advanced Breast Cancer with Difficult Differential Diagnosis of Carcinosarcoma and Atypical Medullary Carcinoma, which had Poor Response to Adriamycin- and Taxane-based Neoadjuvant Chemotherapy: A Case Report
Article information
Abstract
Atypical medullary carcinomas and carcinosarcoma have unique histopathological features. Here we present a case with a breast malignancy that had pathological characteristics of both. A 54-year old patient with a malignant breast mass received 6 cycles of adriamycin-based chemotherapy, followed by 3 cycles of paclitaxel monotherapy, and had a poor clinical response to treatment. A modified radical mastectomy was performed. The pathological diagnosis was complicated by an inability to distinguish between atypical medullary carcinoma and carcinosarcoma. The findings included a tumor that was well-circumscribed, high grade and a syncytial growth pattern as well as biphasic sarcomatous and carcinomatous characteristics. In conclusion, atypical medullary carcinoma and carcinosarcoma of the breast have entirely different prognoses and should be managed differently. Both should be treated by surgical resection, and additional therapy should be considered based on the cancer with the poorer prognosis.
INTRODUCTION
Breast cancer is a heterogeneous disease with various histopathological types. Infiltrating ductal carcinoma (IDC) is the most common type, accounting for 80% of all breast cancers. While many studies have identified the clinical and histological features of IDC, much less is known about less common histological types of breast cancer such as carcinosarcoma, mucinous, tubular and medullary carcinomas. Among these cancers, medullary carcinoma accounts for 1 to 10 percent of invasive breast cancers (1,2); it is characterized by a well-circumscribed, high-grade tumor with a syncytial growth pattern with aggressive infiltration of mononuclear inflammatory cells and absence of an intraductal component. The term 'atypical' medullary carcinoma is used for cases where at least one of the features is missing from the diagnostic criteria (Table 1) (2). Despite their aggressive histological appearance, the prognosis of (atypical) medullary carcinoma appears to be more favorable than that of IDC (3,4).
Another rare form of breast cancer is carcinosarcoma; this tumor accounts for less than 1% of all breast cancers (5,6). It is a form of metaplastic breast cancer with carcinomatous and sarcomatous pathological features. Generally, carcinosarcoma is considered an aggressive tumor with a high risk of recurrence. Moreover, adjuvant therapy is less effective compared to treatment of conventional adenocarcinomas; therefore, optimal treatment for this cancer remains to be defined (7). Atypical medullary carcinoma and carcinosarcoma have unique histopathological features. Here we present a case of malignant breast cancer with pathological characteristics of both types of cancer.
CASE REPORT
A 54-year-old postmenopausal woman was admitted with the complaint of a right breast mass that was noticed 3 months previously. She was taking oral contraceptives for irregular vaginal bleeding over the past 2 years. Menarche occurred at 18 years of age, and her first pregnancy at 26 (Gravida 5 Para 2 Live 2 Death 0 Abortion 3); menopause was at the age of 52. There was no family history of cancer.
On physical examination, there was a palpable, non-tender mass measuring about 5.0 cm in the upper inner quadrant of the right breast. There was no discharge or abnormal skin retraction around the mass. Axillary and cervical lymph nodes were not palpable. The remainder of the examination was within normal limits. Mammography showed a high-density mass without calcification and an indistinct margin in the right breast. Ultrasonography revealed a 5.7 cm-sized mass with three right axillary lymph nodes. The core needle biopsy showed a poorly differentiated carcinoma. Chest CT revealed an oval necrotic mass in the right breast (Fig 1A), and non-enlarged lymph nodes (Fig 1B). The clinical stage was determined to be cT3N0M0 (Stage IIB) according to the AJCC staging system.
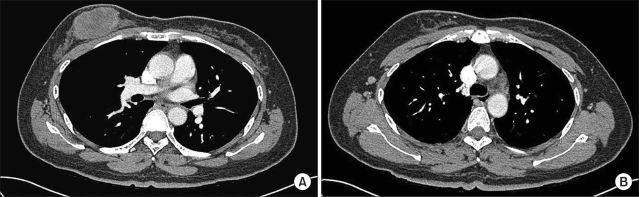
Initial chest CT findings of the patient. There was an oval necrotic mass measuring 6.0 cm in the greatest dimension in the right breast (A) and a small well demarcated lymph node at the right axillary area (B).
Since the tumor-stage of the patient was advanced and the tumor differentiation was very poor, neoadjuvant chemotherapy was started immediately. With the impression of locally advanced breast cancer, the patient received neoadjuvant chemotherapy with a regimen of protracted continuous infusion of 5-fluorouracil (1,000 mg/m2) on days 1~3, combined with bolus injections of adriamycin (40 mg/m2) and cyclophosphamide (600 mg/m2) on day 1 (infusional FAC regimen), which was repeated every three weeks. The patient had grade 3 neutropenia and grade 1 nausea during chemotherapy. After 3 cycles of chemotherapy, the breast mass showed stable disease (SD) by the RECIST criteria and decreased by 20 percent (6.0 cm to 4.8 cm). The patient received an additional 3 cycles of the same regimen. After the sixth cycle, however, the tumor size was nearly unchanged (4.6 cm). Due to the unsatisfactory tumor shrinkage with this regimen, the chemotherapy was then changed to intravenous infusion of paclitaxel (175 mg/m2) every three weeks. Even with 3 cycles of paclitaxel, regrowth of the breast mass (4.6 cm to 4.9 cm) was noted. Predicting that the tumor would no longer be responsive to chemotherapy, we performed a modified radical mastectomy.
The tumor size was 7.0×4.0×4.5 cm without the resection margins. Histopathologically, the specimen consisted of a poorly differentiated carcinoma with spindle cells (Fig 2A). There were frequent multinucleated giant cells noted. Ten axillary lymph nodes were dissected without tumor involvement. The histological grade (HG) of the tumor was III with extensive necrosis (Fig 2B, C). The immunohistochemical staining was negative for the estrogen receptor (ER), progesterone receptor (ER) and c-erbB2, while focally positive for vimentin (Fig 2D). The tumor was diagnosed as a carcinosarcoma (metaplastic carcinoma) based on poorly differentiated carcinoma cells and sarcomatoid spindle cells. In addition, atypical medullary carcinoma was also considered due to the features of a well-defined border, a syncytial growth pattern without tubular differentiation and highly atypical nuclear features with limited lymphoplasmacytic infiltration. Postoperatively, the pathological stage was pT3N0M0 (Stage IIB). The patient received postoperative radiotherapy to the tumor site with a total dose of 50.4 Gy. At 18 months, the patient is doing well without any evidence of recurrence.

Pathological findings of the surgical specimen. The tumor is well defined and shows multinodular growth of oval and spindle tumor cells. There is no evidence of obvious ductal formation or ductal carcinoma in situ. (A, hematoxylin and eosin (H & E) stain, ×10). Tumor cells form solid sheets with geographic coagulative necrosis (B, H & E stain, ×40). The tumor cells are pleomorphic and very large with brisk mitosis (C, H & E stain, ×400). Immunohistochemical staining for vimentin, the fascicled spindle cells are immunoreactive for vimentin (D, DAB, ×200).
DISCUSSION
Both carcinosarcoma and medullary carcinoma are rare types of breast cancer. Carcinosarcoma accounts for less than 1% of all breast cancers, and the incidence has been reported to be 0.8% of all breast malignancies in Korea (5,6). It is a biphasic tumor composed of epithelial and mesenchymal cells. Wargotz et al. reported that carcinosarcoma is a distinct form of metaplastic carcinoma, describing it as a biphasic neoplasm with at least half of the tumor composed of spindle cell components and the remaining area consisting of carcinoma (8). This tumor is usually negative for hormone receptors and c-erbB2 expression. Although the origin of this tumor has not been confirmed, some consider it to develop from myoepithelial cells (8). Clinically, carcinosarcoma presents as a large, single, firm, and rapidly growing mass with infrequent involvement of the axillary lymph nodes. However, it is an aggressive tumor with a high risk of recurrence and a high mortality rate. The recurrence of node-negative carcinosarcoma is around 50~60% (5,8,9). Surgical treatment, radical mastectomy, is the only curative approach. The role of chemotherapy before or after surgery has not been established. One study reported that patients who were treated with neoadjuvant or adjuvant anthracycline-based chemotherapy showed a better clinical outcome compared to those treated with CMF (cyclophosphamide, methotrexate, 5-fluorouracil). However, the neoadjuvant chemotherapy for carcinosarcoma was less effective than for conventional adenocarcinoma (7). The prognosis of patients with carcinosarcoma is poor with the overall 5-year survival rate less than 50% (7~9).
By contrast, medullary carcinoma accounts for 1 to 10 percent of invasive breast cancers (1). It is a well-circumscribed, high-grade tumor with a syncytial growth pattern that has aggressive infiltration of mononuclear cells. There is some inter-observer variability in the diagnosis of this type of cancer (10). The term 'atypical' is added when the medullary tumor lacks one of the conventional diagnostic criteria (2). In our case, there was limited inflammatory cell infiltration while the tumor met the other four diagnostic criteria for medullary carcinoma. The prognosis of medullary carcinoma, whether 'atypical' or typical, is better than that of high grade IDC (11). Studies report the overall 10-year survival rate to be 74% and more than 90% in a group of node negative patients who underwent radical mastectomy (1,2).
Our patient had a large breast cancer with necrosis, no axillary lymph node involvement and poor clinical response to neoadjuvant chemotherapy. The tumor had to be surgically removed, and its pathological diagnosis was both atypical medullary carcinoma and carcinosarcoma. The tumor had all of the distinctive features of both of these rare types of breast cancer. Staining for vimentin had a minimal contribution to the differential diagnosis because both type of cancer can express vimentin (8,12).
With the findings of negative expression of hormones and the c-erbB2 receptor, a so-called triple-negative breast cancer was considered in the differential diagnosis. This type of breast cancer makes up about 15% of all types of breast cancer, and has been morphologically described as metaplastic, atypical or typical medullary or adenoid cystic cancers. The pathological findings in this case showed a high-grade tumor with increased proliferation rate and central necrosis, which are the features of a triple-negative cancer. Although the possibility of a triple-negative breast cancer still exists for this tumor, we did not perform immunoistochemical analysis for CK5/6, EGFR and p53 or BRCA1 mutation testing (13). This type of cancer is characterized by aggressive behavior and no specific systemic regimen is recommended for its treatment. EGFR overexpression or c-KIT expression is often found in triple-negative breast cancer and the tyrosine kinase inhibitors for targeting these signaling pathways are under clinical investigation (13).
The management of a patient with two different pathological findings in the same tumor can be a problem, especially when the findings have different prognostic implications and require different treatments. The prognosis of (atypical) medullary carcinoma is favorable without the need for adjuvant therapy. However, if carcinosarcoma is taken into consideration, this more aggressive tumor requires rigorous follow-up to detect recurrence, and additional post-operative therapy would be indicated. Since the patient, reported here, showed a minimal response to the preoperative chemotherapy with anthracycline and taxanes, adjuvant chemotherapy with the same drugs would not be expected to confer any additional benefits. Instead, radiotherapy to the tumor site was recommended. In one report, carcinosarcoma appeared to benefit from sarcoma-type chemotherapy containing ifosfamide (7). Other possible agents to consider for treatment are gemcitabine, irinotecan and oxaliplatin, which have had no reported proven effect on carcinosarcoma of the breast to date.
In conclusion, atypical medullary carcinoma and carcinosarcoma are rare types of breast cancer. Both require surgical resection. However, when both are present in the same tumor additional treatment should be performed based on the type of cancer with the poorer prognosis.
Notes
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MOST) (R11-2000-082-03002-0).
