A Phase II Study of Weekly Paclitaxel Plus Gemcitabine as a Second-Line Therapy in Patients with Metastatic or Recurrent Small Cell Lung Cancer
Article information
Abstract
Purpose
Paclitaxel (P) and gemcitabine (G) are clinically synergistic in small cell lung cancer (SCLC). We evaluated the efficacy of PG as a salvage treatment for SCLC patients whose disease progressed after a platinum-containing regimen.
Materials and Methods
Eligibility included histologically confirmed SCLC, one dimensionally measurable disease, Eastern Cooperative Oncology Group performance status 0-2, and progressive disease after platinum-based chemotherapy. Treatment consisted of P (80 mg/m2) and G (1,000 mg/m2) on days 1 and 8 of each cycle of 21 days until disease progression.
Results
Thirty-three patients seen between December 2005 and February 2009 were selected into this study. Thirty patients (91%) had received irinotecan-platinum, and three had received etoposide-platinum. Sixteen patients (49%) had a treatment-free interval of less than 3 months. The overall response rate was 30.3% (29.4% in sensitive relapse and 31.3% in refractory relapse). The median time to progression was 12.0 weeks and median overall survival (OS) 31.0 weeks, with a 1-year OS rate of 30.3%. Toxicities were moderate and manageable with 18.2% grade (G) 4 neutropenia, 24.2% G3 thrombocytopenia, 6.1% G3 sensory neuropathy, and 3% G3 asthenia. One patient developed febrile neutropenia.
Conclusion
Second-line paclitaxel and gemcitabine were well-tolerated and moderately active in SCLC patients previously treated with platinum-based chemotherapy.
Introduction
Most patients with small cell lung cancer (SCLC) relapse within a year of initial therapy, and many are candidates for second-line treatment. Although many patients are in excellent physical condition at the time of relapse, few drugs or drug combinations are capable of achieving tumor regression in this setting. Patients with sensitive relapse may respond to a number of agents including topotecan, irinotecan, vinorelbine, paclitaxel, and gemcitabine [1-4]. Topotecan is the best-documented second-line therapy for previouslytreated SCLC, although treatment outcomes have been disappointing. Topotecan produces an 18% response in patients with sensitive relapse, and in refractory patients, the response is less than 10% [5,6]. A topotecan/cisplatin doublet showed a higher response rate in patients with sensitive relapse although median survival times were similar to those obtained with topotecan alone [7]. For some patients with sensitive relapse, re-induction with the same regimen or topotecan might be considered, but for patients with refractory relapse, no regimen is a standard option. Thus, patients with good performance status are candidates for clinical trials for further treatment.
As a single agent, paclitaxel produces a response rate of 27% in SCLC patients with sensitive relapse and a response rate of 20% in those with refractory relapse [8]. Gemcitabine has a response rate of 16% in SCLC patients with sensitive relapse and a response rate of 6%-13% in those with resistant relapse [4,9]. Because of their single-agent activity, different mechanism of action, non-overlapping toxicities, in vitro synergy, and beneficial pharmacologic interaction, the use of a combination of paclitaxel and gemcitabine is an attractive option [10,11]. Paclitaxel significantly decreases the systemic clearance and volume of distribution of gemcitabine, and significantly increased steady-state concentrations of gemcitabine. Interpatient and intrapatient variability in gemcitabine pharmacokinetics was not observed when gemcitabine was administered in combination with paclitaxel in non-small cell lung cancer (NSCLC) patients [11]. Some phase II trials of first-line gemcitabine plus paclitaxel in NSCLC patients showed encouraging response rates of 41%-53% [12-14]. These combination chemotherapies, in which paclitaxel was administered every 3 weeks, were associated with substantial toxicity, and grade 3-4 neutropenia developed in 70%-80% of patients. Weekly paclitaxel treatment has an efficacy comparable to that of the every-3-weekly paclitaxel but with less myelosuppression and neurotoxicity.
Based on these studies, we selected a combination of weekly paclitaxel (80 mg/m2) and gemcitabine (1,000 mg/m2) and conducted a phase II study to evaluate the efficacy and safety of this combination in patients with SCLC who have relapsed or failed to respond to first-line platinum-based therapy.
Materials and Methods
1. Eligibility criteria
Eligibility in the study required histologically- or cytologically-confirmed SCLC that progressed during or after first-line chemotherapy or chemoradiotherapy. Patients needed to have measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) [15]. Additional criteria were as follows: patients must (1) be at least 18 years old; (2) have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2; (3) have not had prior radiotherapy on measurable lesions (but previous surgery and/or chest radiotherapy for the primary lesion was allowed); (4) have had a minimum of 28 days since any prior chemotherapy or radiation before study entry; and (5) have adequate bone marrow, hepatic, and renal functions, defined as a white blood cell count ≥ 3,500/mm3, absolute neutrophil count (ANC) ≥ 1,500/mm3, platelets ≥ 100,000/mm3, alanine aminotransferase or aspartate aminotransferase ≤ 2.5 times the upper normal limit, serum bilirubin < 1.5 times the upper normal limit, and serum creatinine ≤ 1.5 mg/dL. Patients were excluded if there was severe comorbidity such as a myocardial infarction within the preceding six months, symptomatic heart disease (including unstable angina, congestive heart failure, or uncontrolled arrhythmia), or serious concomitant infection, including post obstructive pneumonia. The presence of central nervous system (CNS) metastases, however, was not an exclusion criterion, provided that CNS symptoms were absent or sufficiently minor to be well-controlled with corticosteroids. Written informed consent approved by the Institutional Review Board of the National Cancer Center was obtained from all patients prior to entering the study. The study followed the Declaration of Helsinki and good clinical practice guidelines. This study was registered with ClinicalTrials.gov (identifier: NCT00453 167).
2. Assessment
A complete history and physical examination, including documentation of concomitant medications and performance status, standard laboratory studies, and an electrocardiogram, was performed within 14 days prior to study entry. Chest X-ray, computed tomography scans of the chest including the upper abdomen, magnetic resonance imaging of the brain, and a radionuclide bone scan were performed within 4 weeks prior to study entry. Complete blood cell counts and chemistry were performed on days 1 and 8 of each cycle. Objective tumor response was assessed every two cycles according to the RECIST criteria [15]. Toxicity was graded by the National Cancer Institute Common Toxicity Criteria ver. 3.0.
3. Treatment
Paclitaxel (Padexol, Seoul, Korea) and gemcitabine (Geroam, Seoul, Korea) were administered to patients. Treatment consisted of 80 mg/m2 paclitaxel and 1,000 mg/m2 gemcitabine by intravenous infusion on days 1 and 8 every 3 weeks until disease progression. Paclitaxel was given first as a 60-minute intravenous infusion, immediately followed by a 30-minute gemcitabine intravenously. Premedication consisted of dexamethasone (10-20 mg intravenously), pheniramine maleate (45.5 mg intravenously), and famotidine (20 mg intravenously) before paclitaxel infusion. Granisetron (3 mg) was given intravenously before paclitaxel infusion. During the course of chemotherapy, paclitaxel doses were decreased by 15 mg/m2 for grade 4 hematologic toxicities or grade 3 or 4 non-hematologic toxicities. Gemcitabine doses were decreased by 20% for grade 4 hematologic toxicities or grade 3 or 4 non-hematologic toxicities. The dose reduction was maintained in the subsequent cycles.
4. Statistical analysis
The primary objective of this study was to estimate the overall response rate of paclitaxel and gemcitabine as second-line chemotherapy in patients with metastatic or recurrent SCLC. The secondary objectives were to estimate the time to progression (TTP) and overall survival (OS). Simon’s minimax two-stage design was used to determine the sample size [16]. The undesirable response rate was 20% and the desirable expected response rate was 40%. With 80% power and a 5% one-sided type I error, 18 patients were needed in the first stage, and 33 patients were needed in total. At the first stage, if there were five or fewer responses out of the initial 18 patients, the study would conclude that the anticipated response rate is less than 20% and would terminate. Otherwise, accrual would continue to a full sample of 33 assessable patients. At the second stage, at least 11 objective responses among 33 patients were required for this regimen to be regarded as worthy of further investigation. The response rate of the treatment was calculated as the ratio of the number of complete and partial responders to the total number of evaluable patients. A 95% confidence interval for the response rate was computed based on the binomial distribution function. The toxicity profile was estimated as the ratio of the number of occurrence to the total number of evaluable patients. The TTP and OS were estimated by the Kaplan-Meier method. The OS was defined as the period of time from the first day of treatment to death from any cause. Patients still alive were censored at the last day on which they were known to be alive. The TTP was defined from the first day of treatment to the date that disease progression was assessed. The duration of response for all responders was defined as the period of time from the date of the first response, i.e., either partial response or complete response (whichever occurred first), to the date of disease progression.
Results
1. Patient characteristics
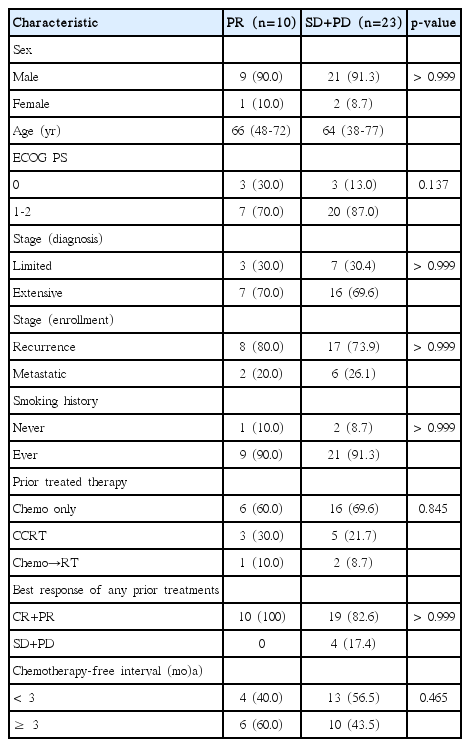
Between December 2005 and February 2009, 33 patients were prospectively enrolled. Patient characteristics are shown in Table 1. The median age was 65 years (range, 38 to 77 years). Thirty patients (91%) were men, and 32 patients (97%) had an ECOG PS of 0 or 1. Three patients (9%) had never smoked. Twenty-three patients (70%) had extensive disease at diagnosis. All patients received platinum doublets. Thirty patients (91%) received irinotecan-platinum and three patients received etoposide-platinum. As a prior therapy, 23 patients received chemotherapy alone, and 10 patients received chemoradiation. Among the chemotherapy-only group, all 23 patients received irinotecan-platinum chemotherapy. Among the 10 patients treated with chemoradiation, seven patients received irinotecan-platinum, and three patients received etoposide-platinum. Sensitive relapse was defined as a chemotherapy-free interval of 3 months or greater, and refractory relapse was defined as no response to initial therapy or progression within 3 months after initial therapy. Sixteen patients (49%) had a refractory relapse.
2. Treatment exposure
The median number of cycles administered was three (range, 1 to 12 cycles), and 10 patients completed six cycles of chemotherapy. Over 133 cycles, delayed chemotherapy administration occurred in 44 cycles (33%), and dose reduction occurred in 22 cycles (16%). The most common cause for the delay in chemotherapy administration was either holiday (23 cycles) or neutropenia (19 cycles). Twenty-two cycles required dose reduction, mainly because of neutropenia (18 cycles), thrombocytopenia (3 cycles), and pneumonia (1 cycle). The average relative dose intensity was 86.4% for paclitaxel and 85.4% for gemcitabine.
3. Efficacy
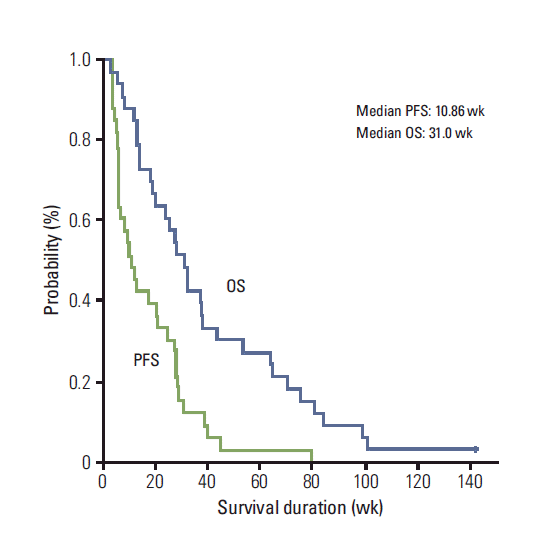
No complete response (CR) was observed. Ten patients achieved partial response (PR). The overall response rate was 29.4% in sensitive relapse and 31.3% in refractory relapse (Table 2). All patients were tracked until death. The median TTP was 12.0 weeks (95% confidence interval [CI], 7.57 to 16.44), the median OS was 31.0 weeks (95% CI, 23.44 to 38.56), and the 1-year OS rate was 30.3% (95% CI, 14.6 to 46.0) (Fig. 1). No pretreatment characteristic, including sex (male vs. female), ECOG PS (0 vs. 1-2), stage at diagnosis (limited vs. extensive), smoking history (never vs. ever), prior treatment (chemotherapy only, concurrent chemoradiotherapy vs. chemotherapy followed by radiotherapy), best response of any prior treatment (PR vs. non-PR), treatment-free interval (< 3 months vs. 3-6 months vs. ≥ 6 months), was predictive of tumor response (Table 3). Twenty patients received salvage chemotherapy after disease progression on PG chemotherapy. The salvage treatments included cyclophosphamideadriamycin-vincristine (6 patients), adriamycin-ifosfamide-vincristine (5 patients), etoposide-cisplatin (3 patients), irinotecan-cisplatin (2 patients), oral etoposide (2 patients), adriamycin-ifosfamide (1 patient), and pemetrexed (1 patient).
4. Toxicity
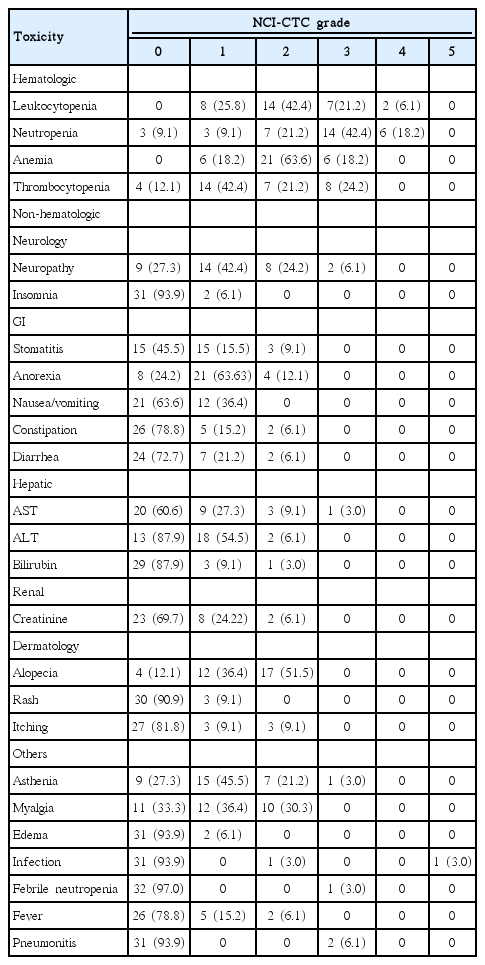
All patients (n=33) who received at least one cycle of therapy were assessable for toxicity. Toxicity was reported as the maximum toxicity experienced during entire study treatment, rather than just during the first cycle. Hematologic and non-hematologic toxicities observed over the entire course of the study are summarized in Table 4. Overall toxicities were moderate and manageable. The most common grade (G) 3 or 4 toxicity was myelosuppression. G4 neutropenia was observed in 18.2% of patients and G3 thrombocytopenia was observed in 24.2% of patients. Only one patient developed febrile neutropenia. Another patient died of pneumonia during the third cycle, but G1 neutropenia was present (ANC, 1,219/μL). G3 sensory neuropathy developed in two patients, and their chemotherapy was stopped. One patient developed G3 neuropathy after the sixth cycle. Another patient developed G3 neuropathy after the first cycle, but recovered completely in 3 months. One patient developed G3 asthenia after the second cycle, but PD was confirmed.
Discussion
This study was a single-arm phase II study to evaluate the efficacy of paclitaxel plus gemcitabine in patients with SCLC that progressed or failed to respond to platinum-based chemotherapy. Historically, the median OS is only 14 weeks if no active therapy is given other than best supportive care (BSC) after first-line chemotherapy [17]. Second-line chemotherapy increased OS and resulted in better symptom control compared with BSC [17]. The strongest predictor of outcome for patients with relapsed SCLC is the duration of remission. Patients with sensitive disease respond to the same initial therapy in approximately 50% of cases. The median OS from the start of a second-line therapy is approximately 6 months. In patients with refractory disease, response rates to second-line therapy are less than 10%, and the median OS from the start of a second-line therapy is 4 months. Response rates to combination chemotherapy are higher than those to a single agent; however, the reported response rates are variable, reflecting the population heterogeneity [18].
Our study began in 2005, and a similar study's report was published in 2006. They showed that weekly paclitaxel and gemcitabine treatment were moderately active in SCLC patients pretreated with platinum and etoposide [19]. Paclitaxel (80 mg/m2) was given on days 1, 8, and 15, and gemcitabine (1,000 mg/m2) was given on days 1 and 8 every 3 weeks. Of these patients, 32% had refractory disease, and the objective response rate was 26%, including 20% in patients with refractory SCLC and 28.6% in patients with sensitive SCLC. The response rate was only slightly higher in patients with sensitive relapse than in those with refractory relapse, indicating that the regimen may be more useful in the former group. Predictive of response to second-line chemotherapy were the interval between the completion of induction and relapse, the extent of tumor regression achieved with the induction regimen, and the composition of the induction program. In the current study, the patient population was different from that previous study. Platinum plus irinotecan has been the standard first-line chemotherapy for SCLC at the National Cancer Center in Korea since 2002, when the Japanese Cooperative Oncology Group showed that irinotecan plus cisplatin was superior to etoposide plus cisplatin in terms of response rate, progression-free survival (PFS), and OS [20]. In this study, 30 patients (91%) received irinotecan plus platinum and three patients received etoposide plus platinum. Sixteen patients (49%) had refractory relapse. Given the refractory nature of the disease, the overall response rate of 30.3% in the current study is remarkable, although we set our initial target response rate to be 40%. Our study design required at least 11 objective responses among 33 patients for this regimen to be regarded as worthy of further investigation; 10 patients achieved PR. We observed similar response rates in both groups (29.4% in sensitive relapse and 31.3% in refractory relapse). These results suggest that this regimen may work in both groups. Also, high activity in patients with refractory disease may reflect a lack of adverse effects of prior treatment with irinotecan/platinum on the antitumor activity of paclitaxel/gemcitabine, which suggests a lack of cross-resistance between paclitaxel/gemcitabine and irinotecan/platinum. A recent randomized phase 3 trial of amrubicin versus topotecan as second-line treatment [21] reported a median OS of 7.5 months with amrubicin versus 7.8 months with topotecan; in refractory patients, median OS was 6.2 and 5.7 months, respectively. Median PFS was 4.1 months with amrubicin and 3.5 months with topotecan. Overall response rate was 31.1% with amrubicin and 16.9% with topotecan [21]. In this study, although amrubicin did not improve survival when compared with topotecan in the second-line treatment of patients with SCLC, OS did not differ significantly between treatment groups and improvement in OS was noted in patients with refractory disease treated with amrubicin. Although direct comparison of phase 2 data with phase 3 data is not reasonable, the weekly paclitaxel/gemcitabine regimen in the current study showed efficacy comparable to amrubicin and topotecan.
Because second-line chemotherapy in SCLC patients is only palliative, we have to consider quality of life, toxicity, and cost. In the second-line setting, all patients had already received platinum doublets, and thus, we had to consider cumulative toxic effects. A randomized phase III trial comparing oral topotecan (2.3 mg/m2/day for 5 days every 21 days) to intravenous topotecan (1.5 mg/m2/day for 5 days every 21 days), found reasonable response rates (18.3% vs. 21.9%) [22]. However, G4 neutropenia was common (47% vs. 64.2%), and diarrhea of all grades was observed (35.9% vs. 19.9%). A study of second-line weekly paclitaxel and gemcitabine showed G3-4 asthenia in 13% of patients [19]. The average relative dose intensity was 72% for paclitaxel and 83% for gemcitabine. In the current study, the dose intensity was 86.4% for paclitaxel and 85.4% for gemcitabine, and treatment-related toxicity was observed considerably less often (18.2% G4 neutropenia and 24.2% G3 thrombocytopenia). Aside from myelosuppression, G3 non-hematologic toxicities were rare, and most of them were manageable with good compliance.
Conclusion
In conclusion, second-line paclitaxel and gemcitabine were well-tolerated with a 30.3% response rate in SCLC patients previously treated with platinum-based chemotherapy. Our study suggests that paclitaxel and gemcitabine can be good options for second-line therapy in platinum-resistant SCLC patients.
Notes
Paclitaxel (Padexol) and gemcitabine (Gero-am) were provided by Shinpoong Pharmaceutical Co., Seoul, Korea.
Acknowledgements
This work was supported by NCC Grants 0510140 and 0810090 from the National Cancer Center, Korea.