Body Mass Index and Doses at Organs at Risk in a Mediterranean Population Treated with Postoperative Vaginal Cuff Brachytherapy
Article information
Abstract
Purpose
Association between body mass index (BMI) and doses in organs at risk during postoperative vaginal cuff brachytherapy (VCB) treatment has not been evaluated. The aim of this study was to analyse the impact of BMI on the dose delivered to bladder and rectum during high-dose-rate VCB using computed tomography (CT) scans at every fraction.
Materials and Methods
A retrospective analysis of 220 planning CT sets derived from 59 patients was conducted. Every planning CT was re-segmented and re-planned under the same parameters. Rectum and bladder dose-volume histogram values (D0.1cc, D1cc, and D2cc) were extracted and evaluated. The mean values for all applications per patient were calculated and correlated with BMI, as well as other factors influencing rectal and bladder doses. Multiple regression analysis performed to model organ at risk dose-volume parameters.
Results
According to World Health Organization (WHO), 6.8% of patients were normal, 35.6% were overweight, and 57.6% were class I obese. Median rectal doses were 133.5%, 110.9%, and 99.3% for D0.1cc, D1cc, and D2cc, respectively. The corresponding median bladder doses were 96.2%, 80.6%, and 73.3%, respectively. BMI did not show significant association with rectal doses. However, BMI did show a significant association with evaluated bladder dose metrics (D0.1cc, r=–0.366, p=0.004; D1cc, r=–0.454, p < 0.001; D2cc, r=–0.451, p < 0.001). BMI was retained in the multivariate regression models (D0.1cc, p=0.004; D1cc, p < 0.001; D2cc, p=0.001).
Conclusion
In this group of Mediterranean, overweight, and moderately obese patients, BMI showed association with lower bladder dose values, but not with rectal doses.
Introduction
Obesity is not only a risk factor for cancer development but also a factor affecting treatment outcomes. Endometrial cancer (EC) was the first malignancy to be recognized as related to obesity. One study suggested that up to 90% of type 1 EC patients are obese [1]. The suggested link between obesity and EC risk is an excess of unopposed oestrogen. However, obesity plays additional roles in EC. It has been associated with lower quality of life values among EC survivors and higher body mass index (BMI), measured before EC diagnosis, has been associated with higher all-cause and endometrial cancer–specific mortality [2].
Early stage EC treatment involves surgery (total abdominal hysterectomy and bilateral salpingo-oopherectomy with or without pelvic lymph node dissection) followed by adjuvant radiotherapy in selected cases. Randomized studies have shown that radiotherapy (RT) reduces the risk of pelvic relapse. Vaginal cuff is the most common site of relapse and the Postoperative Radiation Therapy for Endometrial Carcinoma 2 (PORTEC-2) trial demonstrated that patients with intermediate-risk EC can be treated safely with postoperative vaginal cuff brachytherapy (VCB) in the absence of whole pelvic external beam radiotherapy, thus decreasing toxicity.
Although VCB is one most commonly used adjuvant gynaecological treatments, there is a lack of studies analysing whether dosimetric factors are influenced by overweight. Our aim, therefore, was to evaluate the effect of BMI and perivaginal fat on dose in organs at risk during fractionated VCB.
Materials and Methods
retrospective analysis of 220 consecutive brachytherapy fractions derived from 59 patients who underwent postoperative VCB for gynecological cancer was conducted. Fiftysix patients underwent VCB due to endometrial cancer and three patients due to cervical cancer. Twenty-six endometrial cancer patients underwent postoperative VCB alone using six fractions; the remaining patients were treated with whole pelvis external beam radiotherapy (WPRT) followed by three or four VCB fractions (15 patients underwent each fractionation). All cervical cancer patients underwent VCB as a boost after WPRT. Characteristics of patients, tumour, and treatment are shown in Table 1. Patients were assessed weekly during treatment and every 4 or 6 months afterwards according to the clinical stage. Planning computed tomography (CT) sets were retrieved from the records. Brachytherapy was performed with the largest diameter cylinder that could fit comfortably into the vaginal vault; and the cylinders were positioned to remain parallel to the cranio-caudal axis of the patient. No instructions were given prior to the VCB procedure apart from the need to evacuate prior to coming to the hospital.
1. CT acquisition
All patients underwent a CT planning scan at every application, with 2-mm thick slice at 2-mm overlapping intervals in the supine position using a Foley bladder catheter instilling dilute contrast medium (5 mL of Omnipaque350 [GE Healthcare Bio-Sciences, Madrid, Spain] into 45 mL of saline solution) in order to increase bladder visibility during segmentation and volume reproducibility during treatment.
2. Segmentation and planning
CTs were transferred to a 3D treatment planning system (Oncentra v.4.1, Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden). To improve comparisons, the same physician re-contoured and re-planned every image set under the same conditions for an iridium-192 remote afterloading unit (MicroHDR Nucletron, Nucletron, an Elekta company), regardless of the treatment administered. The entire bladder volume was delineated, and the rectum was defined from 1 cm above the cylinder tip to 1.5 cm below the last activated source dwell position. An active length of 2.5 cm was used to deliver a fraction dose of 5 Gy at 5 mm depth to the vaginal surface. Dose-volume histograms (DVHs) were generated. D0.1cc, D1cc, and D2cc for bladder and rectum were assessed from DVHs for each fraction. The angle of the vaginal cylinder applicator related to the horizontal plane parallel to the craneo-caudal patient axis was calculated, positive values indicating a tip directed to the bladder and negative values indicating a posterior displacement of the cylinder tip toward the rectum. Cylinder size and rectum and bladder volumes were also noted.
3. Fat measurement
BMI was calculated and categorized using the World Health Organization (WHO) definitions. The BMI formula was weight in kilograms divided by the square of the height in metres. Categories were underweight < 18.5 kg/m2; normal 18.5-24.9 kg/mm2; overweight 25.0-29.9 kg/mm2; obese I 30.0-34.9 kg/mm2; obese II 35.0-39.9 kg/mm2; and obese III > 40.0 kg/mm2. We considered fat that was confined within the 100% isodose on the first CT planning scan as the perivaginal fat. It was segmented by thresholding on the Hounsfield units (HU; –190 to –30 HU) and the volume was recorded. Perivaginal fat analysis was performed only in the first CT planning scan because the most common practice for VCB is to perform only one CT and to translate this plan to the next fractions.
4. Statistical analysis
Results are shown as median (interquantile range, IQR). Dose-volume metrics were described as a percentage of the prescription dose. Patient mean dose-volume metrics, volumes, and cylinder angle were calculated from the different fractions. Chi-square tests, Kruskal-Wallis analysis, and univariate regression analysis were performed. A stepwise multiple regression analysis was used to model organs at risk (OARs) DVH parameters as a function of other variables (the significance levels for addition to and removal from the model were set at 0.05 and 0.10, respectively). Analysis of residuals was performed to examine model fit and adherence to regression assumptions. Multicollinearity was assessed using the variance inflation factor. Differences were considered statistically significant at p < 0.05 (two-sided). Analyses were performed using Stata ver. 12 (StataCorp LP, College Station, TX).
Results
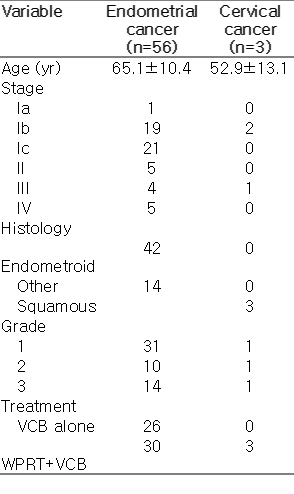
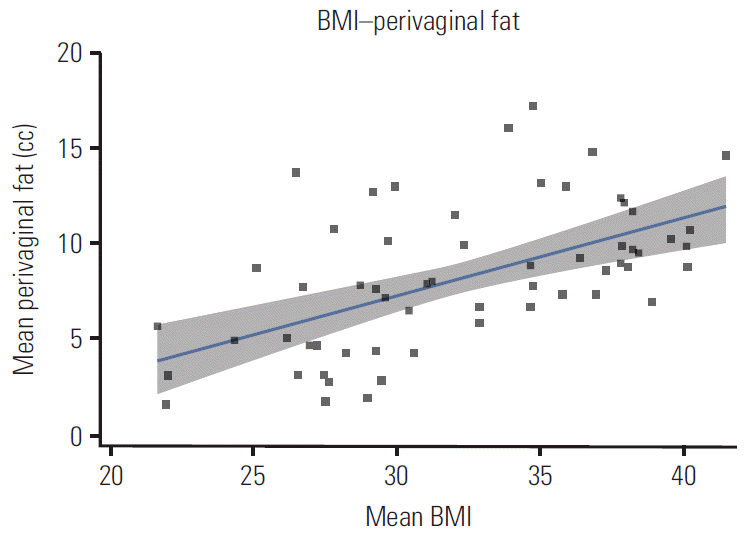
Patient and dosimetric characteristics stratified by WHO BMI classification are shown in Table 2. A mean of 3.7 VCB fractions per patient were retrieved. According to the WHO BMI classification, 6.8% of patients were classified as normal, 35.6% as overweight, and 57.6% as obese I. There were no severely obese patients (class II or III). The median age (IQR) and BMI for the overall group was 64.3 years (18.4) and 32 years (9.5), respectively. Median BMI values for every WHO classification were 22, 27.8, and 36.6 for normal-weight, overweight, and obese I patients, respectively. Patients were significantly older as the BMI class increased (Kruskal-Wallis test, p=0.043). The fact that obese patients were treated with larger cylinder applicators (82.4%) than patients in the normal range (25%) (chi-square test, p=0.037) is significant. A positive, significant correlation was observed between BMI and perivaginal fat volume (r=0.556, p=0.001) (Fig. 1). BMI also showed positive correlation with age (r=0.272, p=0.037) and negative correlation with the bladder DVH metrics (D0.1cc, r=–0.366, p=0.004; D1cc, r=–0.454, p=0.001; D2cc, r=– 0.451, p < 0.001). No correlation was found between BMI and bladder volume. No significant correlation of rectal dose parameters and BMI was observed. A low positive correlation was observed between the perivaginal fat volume and cylinder angle (r=0.288, p=0.027), vaginal cylinders tilted towards the bladder as perivaginal fat volume increased (Fig. 2). Univariate regression showed no significant association between bladder dose metrics and cylinder angle.

Patients' characteristics and rectum and bladder dosimetric parameters according to the World Health Organization body mass index classification
Multivariate regression was performed to determine the effect of variables analysed on the bladder DVH metrics (Table 3). BMI remained significant for every dose bladder metric. Rectum volume was significant only for D1cc and D2cc. Age of patients and the use of a 3.5 cm cylinder diameter showed significance for D2cc. When a reanalysis was performed, replacing BMI with perivaginal fat volume, models were unable to retain the perivaginal fat volume among the predictors.
Patients developed grade 1 or 2 acute toxicity, no higher acute toxicity levels were observed. Late toxicity was uncommon, only one patient developed G3 bladder toxicity and two patients developed late rectal toxicity, one of them G1 and the other G2.
Discussion
The main finding from this study is that higher BMI values showed an association with lower bladder dose parameters but not with rectal dose. Adipose tissues are commonly classified as subcutaneous or visceral fat, the latter being associated with metabolic disease. Visceral fat located in the periprostatic area has been associated with prostate cancer aggressiveness and its volume showed direct correlation with BMI [3]. A reduction in rectal doses among patients with higher BMI during prostate brachytherapy was reported despite not translating into toxicity [4]. This dose reduction is considered to be a result of the inverse square law due to the increase in fatty tissue at the prostate-rectum interface. Our results, from an analysis of a large sample and a high number of brachytherapy insertions, confirm those of a recent study that reported higher bladder doses in women with lower BMI during VCB [5]. A positive, significant correlation was observed between BMI and perivaginal fat volume (r=0.5561, p=0.001) (Fig. 1). Studies have reported correlation between BMI and regional measures of adiposity [6,7]. Unfortunately, due to the limited field of view (FOV) used during CT scan acquisition, we were unable to determine whether correlation existed with visceral fat. The small FOV left some part of the visceral fat outside the scanning area precluding this analysis. Our results do not support an increase in perivaginal fat or application of the inverse square law as the main cause of lower doses in OARs, due to the inability of perivaginal fat to remain in the multivariate regression models. If that was the main explanation, rectal doses would also decrease with higher perivaginal fat volumes. Our data did not show an effect of obesity on rectal dose.
Obesity and overweight have been associated with incidence of cancer and overall mortality. Obesity class I patients are strongly associated (hazard ratio, 3.93) with EC [8], but no association with stage or grade of disease was found in the large Women’s Health Initiative study [9]. The relative risk of death from cancer among women with a BMI of 32 or higher was 2.1 compared with the risk for women with BMI <19 [10].
Although obesity is described as a risk factor for adverse outcomes after treatment for many malignancies, the effect of BMI on EC treatment outcomes has been a subject of debate. While analysis of the National Institutes of Health-AARP Diet and Health Study demonstrated an increased risk of overall and disease-specific mortality among women with higher pre-diagnosed BMI [11], the analysis of the GOG 99 study data associated obesity with higher mortality from causes other than EC but not disease recurrence [12]. The analysis of the ASTEC trial failed to find an association of BMI with a reduced post-treatment survival [13]. A recent systematic review did not suggest an association between BMI and the recurrence of cancer [14].
Vaginal brachytherapy (VBT) has been one of the main adjuvant treatments for EC since the results of randomized trials that showed VBT was not inferior to pelvic irradiation in high intermediate risk patients, and its use is extended after external beam radiotherapy in high risk patients [15]. There have been concerns regarding the methods for delivery of VBT, whether to use a single plan approach or a customized plan at every fraction [16]. The first approach requires some level of homogeneity between insertions because several variables can affect dose deposition to OARs [17-20]. Our data are not influenced by bladder filling due to the routinely placed bladder catheter. Perivaginal fat volume was associated with an anterior cylinder tilt which, paradoxically, did not go with higher bladder dose; therefore, we believe that anatomical visceral fat distribution could be the cause of differences in bladder dose according to BMI (Fig. 3).
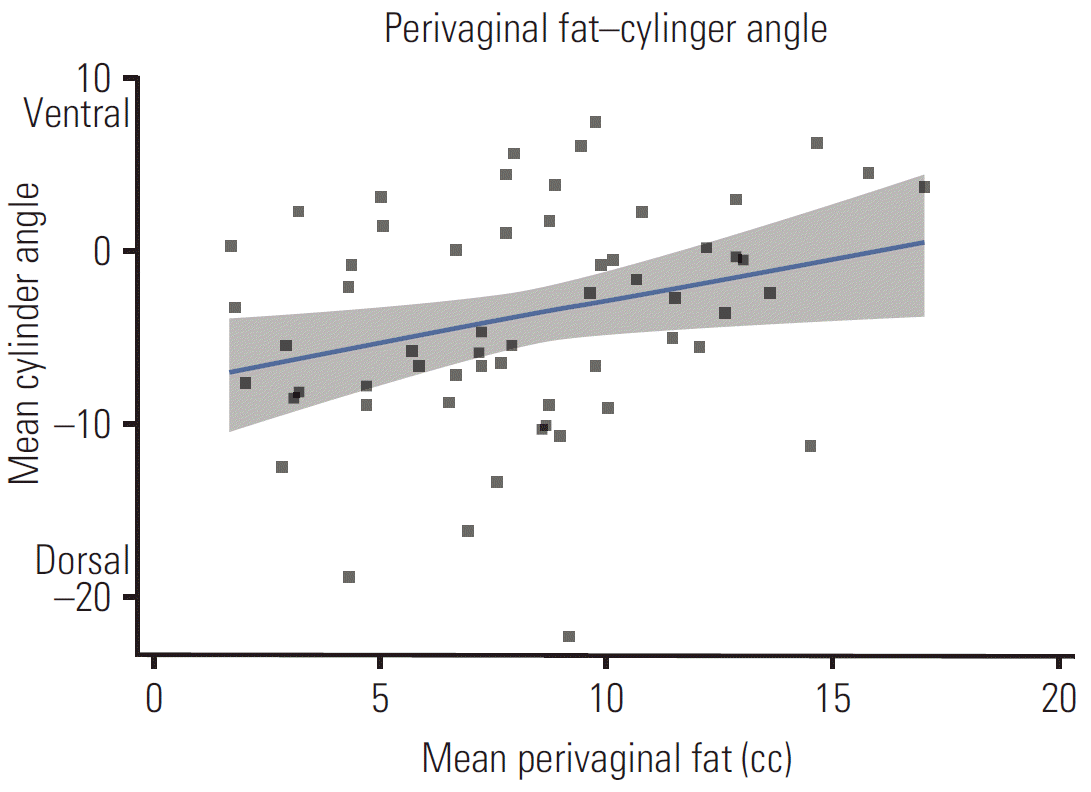
Relationship between fat distribution, bladder, and 100% isodose after a fat pixel intensity transformation. Fat pixels (Hounsfield units, –190 to –30) are shown as white dots. The shaded circle around the vaginal cylinder depicts the 100% isodose. Computed tomography images depict patients with a similar perivaginal fat volume but with different bladder doses (D1cc values as the percent of the prescribed dose). (A) Perivaginal fat volume 4.3 cc, bladder D1cc=74.58%. (B) Perivaginal fat volume 4.4 cc, bladder D1cc=98.3%.
A limitation of our study is the lack of a complete pelvic visceral fat segmentation, which was not possible due to the limited pelvic volume scanned in some insertions. Strong correlation was observed between perivaginal fat volume, which was used as a surrogate for visceral fat volume, and BMI. However, visceral fat volume has not been validated. This measure has not been validated, abdominal adiposity at the umbilicus level being the usual measure of visceral and subcutaneous fat. To study these relationships we are planning visceral fat segmentation at different abdominal and pelvic levels. The fact that there were no severe or extremely obese patients (BMI obese class II and III) in this report is noteworthy, and reflects the BMI distribution among the general population in Mediterranean countries compared with the population in the United States. In addition, the population attributable fraction is greater for the US population (56.8%) than for the European population (45.2%) [21]. Population differences in BMI obese classes II or III could modify these results. Although the study did not focus on clinical results, we believe that is important to know, as much as possible, which variables influence the absorbed doses to organs at risk. This knowledge is more important in a clinical setting where only the first VBT application is planned and the remaining are assumed to be similar due to a fixed geometry, according to the American Brachytherapy Society consensus [22].
Conclusion
In conclusion, the results reported in this study demonstrate a significant inverse correlation between BMI and bladder dose deposition during VCB. The impact of these findings on late toxicity needs to be evaluated in clinical practice.
Notes
Conflict of interest relevant to this article was not reported.