Prognostic Value of Splenic Artery Invasion in Patients Undergoing Adjuvant Chemoradiotherapy after Distal Pancreatectomy for Pancreatic Adenocarcinoma
Article information
Abstract
Purpose
The purpose of this study was to evaluate the outcome of adjuvant chemoradiotherapy (CRT) after distal pancreatectomy (DP) in patients with pancreatic adenocarcinoma, and to identify the prognostic factors for these patients.
Materials and Methods
We performed a retrospective review of 62 consecutive patients who underwent curative DP followed by adjuvant CRT between 2000 and 2011. There were 31 men and 31 women, and the median age was 64 years (range, 38 to 80 years). Adjuvant radiotherapy was delivered to the tumor bed and regional lymph nodes with a median dose of 50.4 Gy (range, 40 to 55.8 Gy). All patients received concomitant chemotherapy, and 53 patients (85.5%) also received maintenance chemotherapy. The median follow-up period was 24 months.
Results
Forty patients (64.5%) experienced relapse. Isolated locoregional recurrence developed in 5 patients (8.1%) and distant metastasis in 35 patients (56.5%), of whom 13 had both locoregional recurrence and distant metastasis. The median overall survival (OS) and disease-free survival (DFS) were 37.5 months and 15.4 months, respectively. On multivariate analysis, splenic artery (SA) invasion (p=0.0186) and resection margin (RM) involvement (p=0.0004) were identified as significant adverse prognosticators for DFS. Also, male gender (p=0.0325) and RM involvement (p=0.0007) were associated with a significantly poor OS. Grade 3 or higher hematologic and gastrointestinal toxicities occurred in 22.6% and 4.8% of patients, respectively.
Conclusion
Adjuvant CRT may improve survival after DP for pancreatic body or tail adenocarcinoma. Our results indicated that SA invasion was a significant factor predicting inferior DFS, as was RM involvement. When SA invasion is identified preoperatively, neoadjuvant treatment may be considered.
Introduction
Pancreatic cancer is one of the most aggressive tumors and its prognosis remains dismal. At presentation, more than 80% of patients are not eligible for curative resection with metastatic or locally advanced disease, and only minorities of patients who undergo surgery survive to approximately 15 months [1]. Major reasons of treatment failure were frequent local recurrence and distant metastasis, even after undergoing curative resection [2].
The early Gastrointestinal Tumor Study Group (GITSG) and European Organization for Research and Treatment of Cancer (EORTC) trials demonstrated improved survival with adjuvant chemoradiotherapy (CRT), but consensus on the benefit of CRT was challenged further by ESPAC-1 study [3-5]. After that, CONKO-001 study showed the efficacy of adjuvant gemcitabine, but the rate of locoregional failure was unsatisfactory with chemotherapy alone [6,7]. Given these observations, many studies have also evaluated the additional role of adjuvant radiotherapy (RT). However, a clear benefit in terms of survival has not been described thus far due to inconsistent results [8-12].
Meanwhile, few studies separately reported on the outcomes of the body or tail adenocarcinoma of the pancreas. Generally, patients with pancreatic body or tail tumors tend to be diagnosed and presented in advanced stage compared with pancreatic head tumors, mainly due to absence of symptoms, such as obstructive jaundice or abdominal pain [13,14]. Limited data is available concerning the role of adjuvant therapy for distal tumors after curative resection, mostly distal pancreatectomy (DP). Unfortunately, previous representative trials of adjuvant CRT had mostly dealt with the outcomes of patients with pancreatic head lesions. Therefore, further studies are needed to examine the role of adjuvant CRT in distal pancreatic cancer. In this study, we retrospectively analyzed the outcomes of adjuvant CRT after DP in a single institution.
Materials and Methods
1. Study population
Between January 2000 and December 2011, a total of 109 patients underwent DP with the aim of achieving cure for adenocarcinoma of the body or tail of the pancreas. Of these patients, 22 who corresponded to the following conditions were excluded patients who were found to have metastatic disease during the pre-adjuvant therapy workup (n=12), who was not recorded about their adjuvant therapy (n=6), who received neoadjuvant CRT (n=3), and who died within 60 days of surgery (n=1). Upon exclusion, the remained cohort included 87 patients. Among them, 13 did not receive adjuvant therapy, 7 received adjuvant chemotherapy only, and 5 underwent CRT at an outside hospital. As a result, the final study cohort included 62 patients. The medical records were reviewed retrospectively, and institutional review board approval was obtained.
Preoperative evaluation of disease extent included computed tomography (CT), ultrasonography, magnetic resonance imaging (MRI), or positron emission tomography (PET). Splenic vessel involvement is retrospectively assessed using a preoperative CT or MRI by a single radiologist, who was blinded to the clinical outcomes. Splenic vessel invasion was radiologically defined as any sign of direct vessel invasion, encasement, or abutment.
In terms of surgical techniques, all patients underwent DP with regional lymph node (LN) dissection and splenectomy. If the tumor invaded the adjacent organs, including stomach, transverse colon, left adrenal gland, or superior mesenteric vein, these structures were also resected to obtain negative margins as possible.
Pathologic characteristics, including tumor size, differentiation, T stage, resection margin (RM), LN status, microscopic angiolymphatic (ALI), venous (VI), and perineural invasion (PNI), were obtained from the pathology report. RM involvement was defined as the presence of tumor cells at the inked margin. Tumor and nodal status were determined using standard TNM staging in accordance to the American Joint Committee on Cancer (AJCC), 7th edition. Great vessel invasion, including splenic artery (SA) and splenic vein (SV), was not routinely described in the pathology report during the study period. Therefore, we used a radiologic definition of splenic vessel invasion (as described above) as a potential prognostic factor.
2. Adjuvant treatments
Nine patients received two cycles of adjuvant gemcitabine plus cisplatin followed by CRT. Other 53 patients underwent adjuvant CRT first at a median 42 days after surgery (range, 27 to 69 days). A three-dimensional conformal RT technique was used in 58 patients (93.5%). Three patients were treated with conventional technique and one patient underwent intensity-modulated RT.
Adjuvant RT was delivered to the tumor bed and regional nodal areas, including celiac, superior mesenteric, peripancreatic, and para-aortic LN’s. Splenic hilar nodes were not included in the target volume. The median total dose was 50.4 Gy (range, 40 to 55.8 Gy), with fractions of 1.8-2.0 Gy once daily. In 11 patients, a total dose of 40 Gy was delivered using 2 Gy/fraction with a 2-week planned rest after 20 Gy. Concomitant 5-fluorouracil (5-FU) was administered for the first 3 days of each 2 weeks of RT. Other 51 patients received RT without planned rest (5 days a week), and concurrent chemotherapy regimen was 5-FU in 31 patients (60.8%), gemcitabine in 16 (31.4%), and capacitabine in 4 (7.8%). All patients completed the planned RT except two patients. One patient missed the last one fraction due to acute gastrointestinal complication, and the other patient omitted the last two fractions because of poor general condition. The median duration of RT was 39 days (range, 32 to 51 days).
Maintenance chemotherapy was administered to 53 patients (85.5%) after the completion of concurrent CRT: 31 patients received 5-FU, 20 received gemcitabine, and 2 received oral enteric coated tegafur-uracil. Maintenance chemotherapy was not offered to 9 patients due to the following reasons: disease progression (n=4), poor performance after adjuvant CRT (n=2), patient’s refusal (n=1), and unknown (n=2).
3. Evaluation of toxicity and survival
Hematologic toxicities caused by chemotherapy were assessed by the criteria set forth by World Health Organization (WHO). During RT, clinical toxicity evaluations were performed once weekly by radiation oncologists. Gastrointestinal toxicities were graded according to the Radiation Therapy Oncology Group (RTOG) acute radiation morbidity scoring criteria.
After the completion of treatment, patients were followed up regularly. Abdominal CT and/or ultrasonography and/or PET were performed every 2-6 months.
Failure sites were classified as locoregional (adjacent nodal, around the remnant pancreas or reappearance of the disease in the surgical bed), distant (liver, non-regional LN’s, peritoneum, or systemic), or both. Overall survival (OS) was calculated from the date of surgery to the date of death from any cause or the last follow-up. Disease free survival (DFS) was defined as the time interval between the date of surgery and any failure after adjuvant treatment or death.
4. Statistical analysis
The OS and DFS were determined through the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards model was used in a multivariate analysis to estimate the hazard ratio and to adjust the potential confounding effects. The factors found to be significant on an univariate analysis or thought to be clinically important were subjected to a multivariate analysis. p < 0.05 was considered statistically significant. Data were analyzed using SPSS release ver. 18.0.1 (SPSS Inc., Chicago, IL).
Results
1. Characteristics
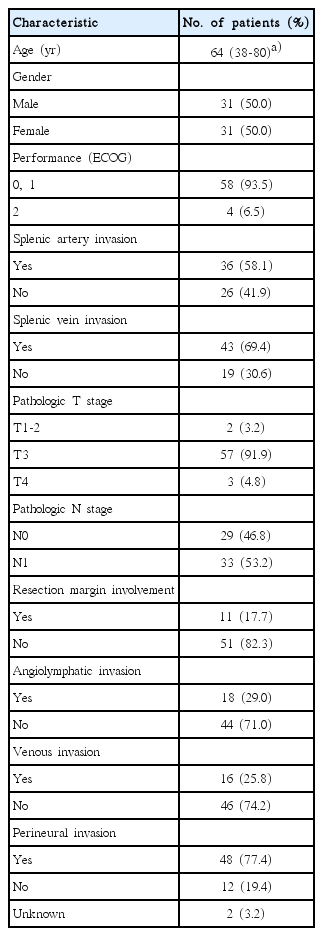
The patient characteristics are shown in Table 1. There were 31 men (50.0%) and 31 women (50.0%) with the median age of 64 years (range, 38 to 80 years). Diagnostic imaging included abdominal CT (n=62), pancreatobiliary MRI (n=39), and/or whole-body PET (n=25). In preoperative imaging, SA and SV invasions were present in 36 (58.1%) and 43 (69.4%) patients, respectively. All patients underwent LN dissection. The median number of examined LN was 10 (range, 3 to 41). Nine patients underwent combined resection of the adjacent organs (2 stomach, 2 left adrenal gland and stomach, 1 portal vein, 1 transverse colon, 1 superior mesenteric vein, 1 mesocolon, and 1 omentum).
Pathologically, tumors were identified as well-differentiated adenocarcinoma in 5 patients (8.1%), moderately differentiated adenocarcinoma in 48 (77.4%), and poorly differentiated adenocarcinoma in 3 (4.8%). The information on histologic differentiation was unavailable in 6 (9.7%). According to the TNM system, 1 (1.6%), 1 (1.6%), 57 (91.9%), and 3 (4.8%) patients were diagnosed with T1, T2, T3, and T4 disease, respectively. Thirty-three patients (53.2%) had LN metastases and the median number of positive nodes was 3 (range, 1 to 9). The median tumor size was 3 cm (range, 0.6 to 7.5 cm). RMs were microscopically involved by the tumor cells in 11 patients (17.7%). Histologic ALI, VI, and PNI were identified in 18 (29.0%), 16 (25.8%), and 48 (77.4%) patients, respectively. Patients’ performance status was assessed in accordance to the Eastern Cooperative Oncology Group (ECOG) criteria. At the initiation of adjuvant therapy, ECOG performance score was 0 in 5 patients (8.1%), 1 in 53 (85.5%), and 2 in 4 (6.5%), respectively.
2. Patterns of failure
With the median follow-up period of 24 months (range, 2 to 107 months), 40 patients (64.5%) experienced relapse. As the first site of relapse, isolated locoregional recurrence (LRR) developed in 5 patients (8.1%). Distant metastasis (DM) occurred in 35 patients (56.5%), 13 (21.0%) of whom had both LRR and DM. Most frequent DM site was the liver (n=18). Other DM developed in the peritoneum (n=8), lung (n=5), infrarenal para-aortic LN’s (n=2), pleura (n=1), and abdominal wall (n=1).
3. Survival and prognostic factors
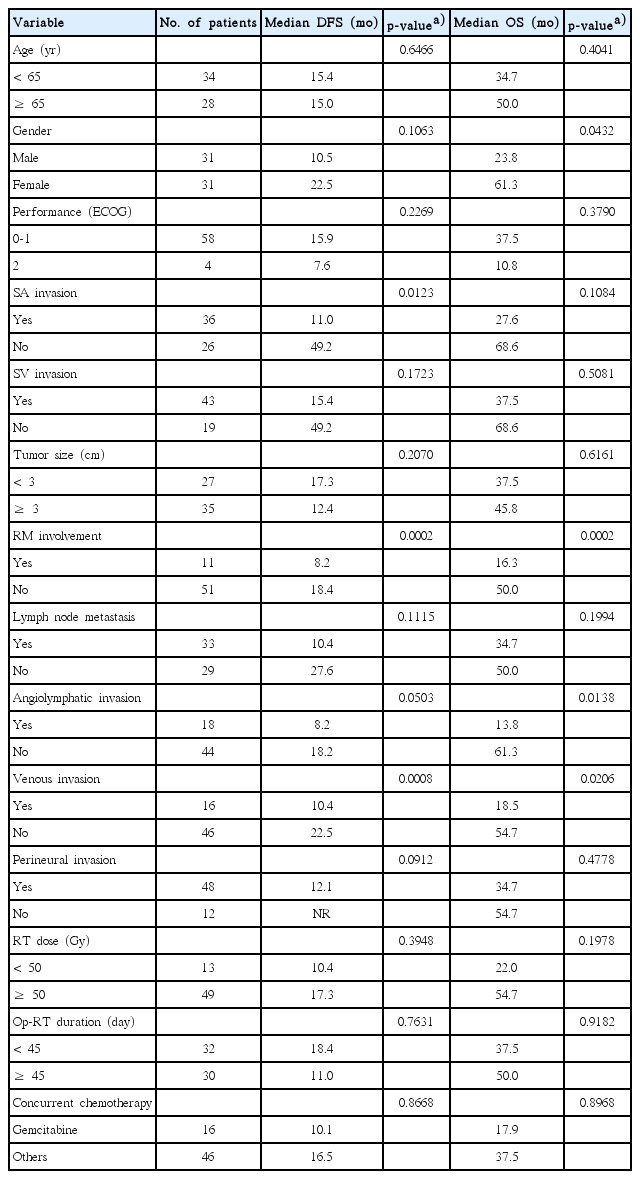
Median OS and DFS for the entire study population were 37.5 months and 15.4 months, respectively. One-, 2-, and 5-year OS was 80.6%, 60.8%, and 36.9%, respectively. Twenty-eight patients were alive and 8 patients had survived for more than 5 years, of whom 5 had no evidence of disease until the last follow-up. One-, 2-, and 5-year DFS was 58.1%, 38.5%, and 25.0%, respectively. The results of univariate and multivariate analyses of OS and DFS are shown in Tables 2 and 3.
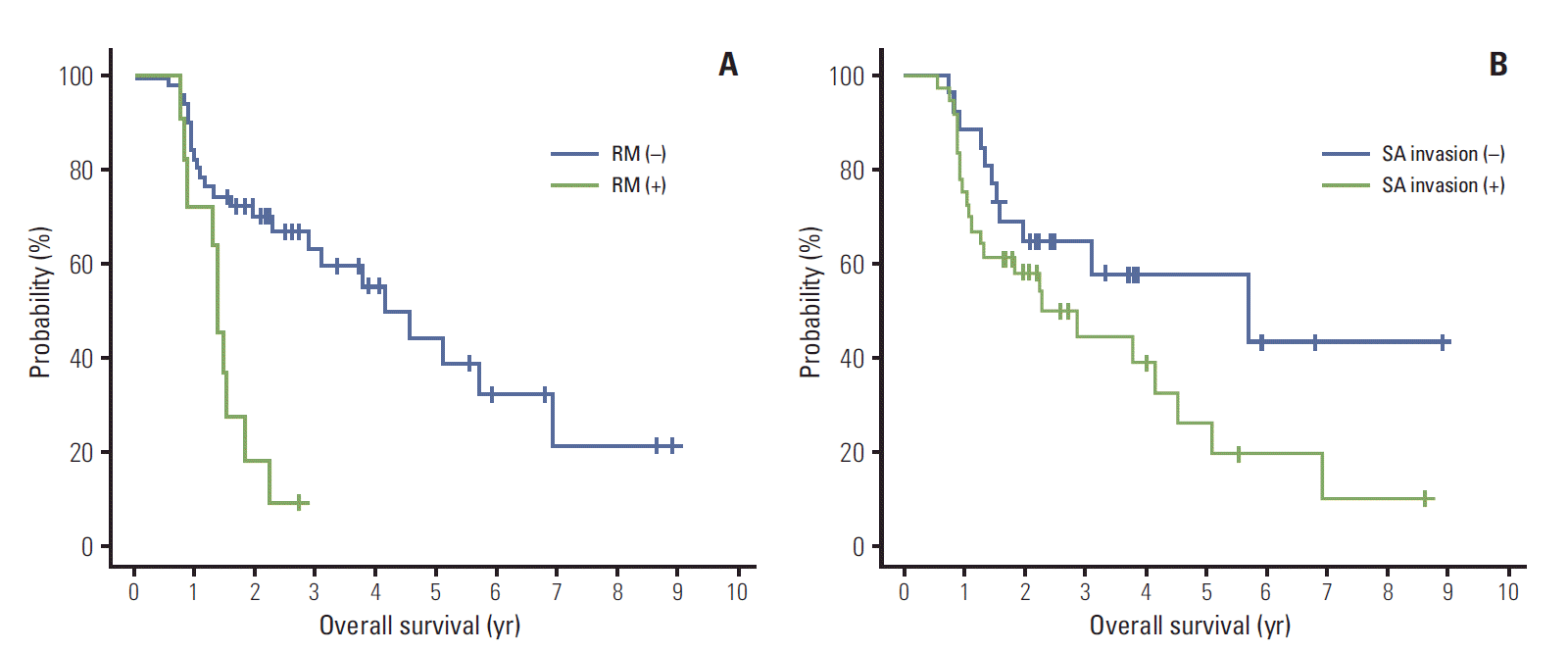
On the univariate analysis, SA invasion, RM involvement, and VI were identified as significant adverse prognosticators for DFS. Among these variables, SA invasion and VI were closely correlated when assessed with the chi-square test (p=0.029), whereas RM involvement was not correlated with the other two variables (RM involvement vs. SA invasion, p=0.794; RM involvement vs. VI, p=0.902). Due to the concerning issue of multicollinearity, we excluded VI in the multivariate model. In addition, LN metastasis was entered into the multivariate analysis as an established prognostic indicator, although it did not significantly affect DFS within our cohort in the univariate analysis. Multivariate analysis revealed that RM involvement (p=0.0004) (Fig. 1A) and SA invasion (p=0.0186) (Fig. 1B) were independent prognostic factors for DFS.
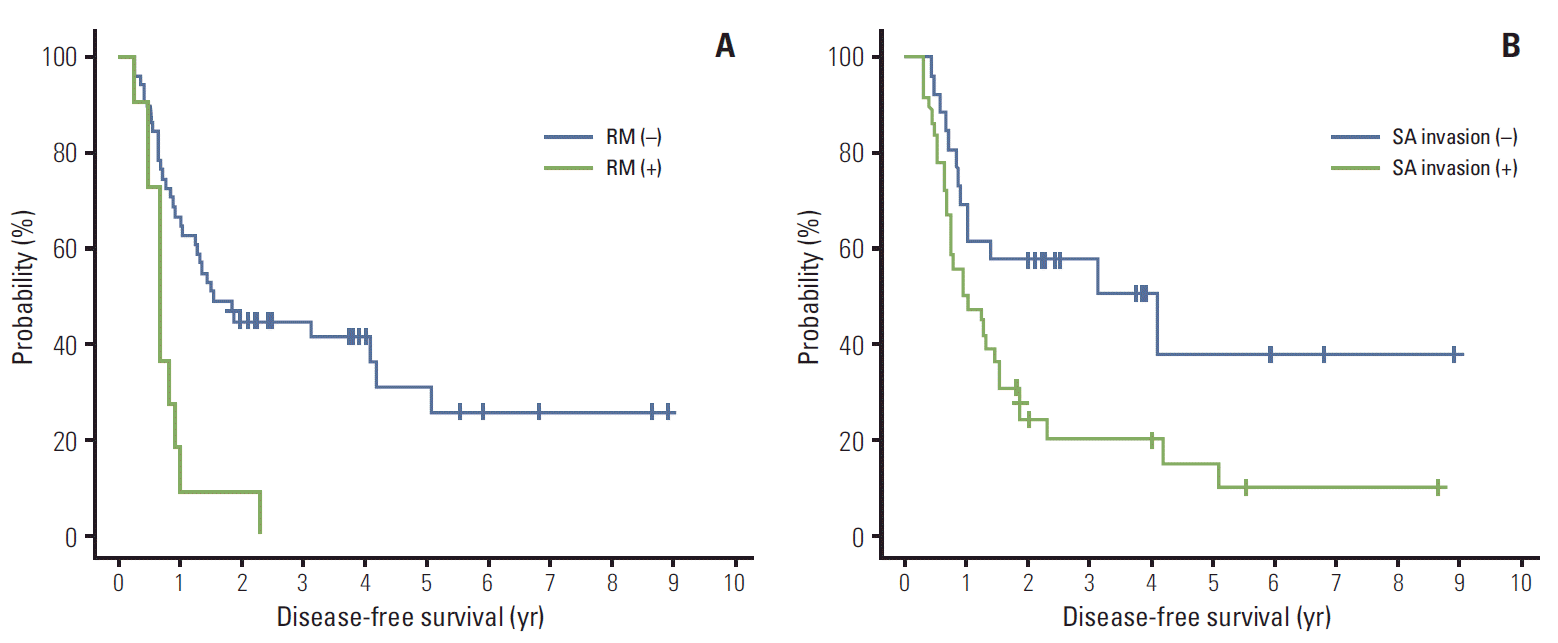
Disease-free survival curves according to the resection margin (RM) involvement (A) and splenic artery (SA) invasion (B).
Also, four factors appeared to be associated with poor OS in the univariate analysis: male gender, RM involvement, ALI, and VI. Along with the preceding results of the correlation test, SA invasion, ALI, and VI were closely correlated with one another (SA invasion vs. ALI, p = 0.044; ALI vs. VI, p=0.032). Instead of ALI and VI, we evaluated the SA invasion in the multivariate model combined with LN metastasis in the same manner of DFS. On the multivariate analysis, RM involvement (p=0.0007) (Fig. 2A) and male gender (p=0.0325) were associated with a significantly poor OS, whereas SA invasion (p=0.3017) (Fig. 2B) and LN metastasis (p=0.2238) were not.
4. Treatment toxicities
During CRT, acute gastrointestinal toxicity occurred in 48 patients (77.4%), with RTOG grade 1 in 15 (24.2%), grade 2 in 30 (48.4%), and grade 3 in 3 (4.8%). Most side effects were nausea, anorexia, and abdominal discomfort, which were relieved in all patients with supportive management. No grade 4 or more toxicity occurred during and after RT. In the course of adjuvant chemotherapy, WHO grade 3 or higher hematologic toxicities were observed in 14 patients (22.6%).
Discussion
The main purpose of this retrospective study was to evaluate the outcomes of adjuvant CRT after DP. To date, there has been no randomized controlled trial to validate the role of adjuvant CRT for pancreatic body or tail carcinoma. Given the paucity of evidences, most preferred adjuvant treatment at our institution was concurrent CRT during the period that our study patients were treated. Thus, we could not include an observation group, because only 13 patients did not receive adjuvant treatment in the same period; as such, the statistical power would not be sufficient for detecting significant differences. Therefore, the impact of adjuvant CRT would be evaluated through a comparison with the other contemporary series.
Some reports found that only about 10% of patients with distal pancreatic cancer underwent surgical resection with curative intent [15,16]. Even in the minority with resectable disease, poor prognosis was expected. Many surgical studies reported that the 5-year OS rates after DP were 10% to 22% [13-16]. In the current study, the 5-year OS rate was 36.9%, which was much higher than the previous surgical reports. It is encouraging, especially considering the high-risk features presented by most of our patients (T3-4 tumors 97%, N1 53%).
Few studies have analyzed the outcomes of adjuvant CRT in patients with adenocarcinoma of distal pancreas. The largest study that has evaluated the impact of adjuvant CRT is from Johns Hopkins Hospital [17]. Redmond et al. [17] reported that adjuvant CRT did not increase survival compared with surgery alone, although they suggested that patients with LN metastases may benefit. Median survival of the adjuvant CRT group was only 16.7 months, which failed to show statistically significant difference compared to those of the surgery alone group (12.1 months). Like the preceding surgical outcomes, this survival was also lower than ours (37.5 months), despite the similar N1 disease ratio (55% vs. 53%). The unsatisfactory survival of the CRT group from Johns Hopkins Hospital may have been influenced by a heterogeneous population that consisted of patients from a broad inclusion period (1985-2006) and multi-institutional adjuvant treatment; our cohort on the other hand was rather homogeneous within a relatively short period (2000-2011), especially from a single institution.
There may be several interpretations for favorable survival observed in our study. First, 94% of the patients had an ECOG performance score of 0 or 1; healthier patients may have been included. Second, the RM involvement ratio (18%) was slightly lower than other studies (about 30%) [13-17]. Furthermore, most patients completed CRT in our cohort and 86% of them also received maintenance chemotherapy. Treatment completion might have affected prolonged survival and some authors also suggested that additional chemotherapy after CRT may be associated with improved survival for resected pancreatic cancer [11,18]. The aforementioned factors may have contributed to promising results in our study. However, this study was based on a small number of patients, and therefore, the possibility of other hidden biases influencing the treatment results may not be completely ruled out.
Commonly, local control of pancreatic body or tail adenocarcinoma after DP has not been separately mentioned. Although many studies included both pancreatic head carcinoma and pancreatic body or tail carcinoma, reported rates of local failure were 35%-62% even after curative resection followed by adjuvant CRT [3-5]. In our study, LRR occurred in 29% (18/62) and isolated LRR developed in only 8% (5/62) of the entire patient population as the first site of relapse. In terms of local control, the results of the present study are impressive, along with the RTOG 97-04 study, which demonstrated the lowest local recurrence rate ever reported among the previous well-known adjuvant trials for pancreatic adenocarcinoma. In the RTOG 97-04 study, LRR of the distal tumor was not separately reported. However, the distribution of relapse was similar regardless of tumor location: in the gemcitabine arm, LRR was 32% in all patients and 33% in patients with pancreatic head tumors; and in the 5-FU arm, LRR was 38% in both groups [8]. Still, it is not clear whether the low rate of LRR was associated with adjuvant CRT, because there is no observation arm in our study and the RTOG 97-04 study. Further studies are needed to elucidate the role of adjuvant CRT in distal pancreatic cancer.
RM involvement has been identified in the literature as a significant prognostic factor for survival in pancreatic adenocarcinoma [10,12,19]. The multivariate analysis in our study confirms these findings. Additionally, it has also been reported that SA invasion is a determinant of poor survival [20,21]. Kanda et al. [20] reported that the invasion of SA, but not that of SV, was one of the most important factors predicting survival in distal pancreatic cancer. Although no consistent definition of SA invasion was described, these results are in close agreement with our study. Furthermore, to the best of our knowledge, this is the first study that showed the association between SA invasion and poor DFS in the adjuvant CRT setting. Even though our definition of SA invasion was based on preoperative imaging (not pathologically proven), we thought that radiological finding before operation have greater clinical implications for determining optimal treatment strategies, rather than postoperative pathologic features. When SA invasion is identified preoperatively, neoadjuvant approach may have a role in saving these patients from unnecessary treatment because more frequent treatment failures (mostly, distant metastases) would be expected despite aggressive surgery followed by CRT.
The current study has several potential limitations. First, due to the nature of retrospective studies, it is possible that other biases may exist, affecting the treatment outcomes. For instance, chemotherapy regimens and sequences varied among patients. Some patients received adjuvant chemotherapy first followed by CRT, although survival was not significantly different between these patients and others (data not shown). Second, a small number of patients also restricts the statistical power. However, with a scarcity of data regarding adjuvant CRT after DP, our study may bring some insights to the treatment of this disease.
Conclusion
In conclusion, postoperative adjuvant CRT may improve survival after DP for pancreatic body or tail adenocarcinoma, with acceptable toxicity. It may come from a low rate of LRR and consequential long term survivors. Our results also indicated that SA invasion was a significant factor predicting inferior DFS, as was RM involvement. In the case of SA invasion in preoperative imaging, neoadjuvant treatment may be considered. Further prospective studies with a large sample size are needed to draw definitive conclusions.
Notes
Conflict of interest relevant to this article was not reported.