Patterns of Failure Following Multimodal Treatment for Medulloblastoma: Long-Term Follow-up Results at a Single Institution
Article information
Abstract
Purpose
The purpose of this study is to investigate the long-term results and appropriateness of radiation therapy (RT) for medulloblastoma (MB) at a single institution.
Materials and Methods
We analyzed the clinical outcomes of 106 patients with MB who received RT between January 1992 and October 2009. The median age was 7 years (range, 0 to 50 years), and the proportion of M0, M1, M2, and M3 stages was 60.4%, 8.5%, 4.7%, and 22.6%, respectively. The median total craniospinal irradiation (CSI) and posterior fossa tumor bed dose in 102 patients (96.2%) treated with CSI was 36 Gy and 54 Gy, respectively.
Results
The median follow-up period in survivors was 132 months (range, 31 to 248 months). A gradual improvement in survival outcomes was observed, with 5-year overall survival rates of 61.5% in 1990s increasing to 73.6% in 2000s. A total of 29 recurrences (27.4%) developed at the following sites: five (17.2%) in the tumor bed; five (17.2%) in the posterior fossa other than the tumor bed; nine (31%) in the supratentorium; and six (20.7%) in the spinal subarachnoid space only. The four remaining patients showed multiple site recurrences. Among 12 supratentorial recurrences, five cases recurred in the subfrontal areas. Although the frequency of posterior fossa/tumor bed recurrences was significantly high among patients treated with subtotal resection, other site (other intracranial/spinal) recurrences were more common among patients treated with gross tumor removal (p=0.016). There was no case of spinal subarachnoid space relapse from desmoplastic/extensive nodular histological subtypes.
Conclusion
Long-term follow-up results and patterns of failure confirmed the importance of optimal RT dose and field arrangement. More tailored multimodal strategies and proper CSI technique may be the cornerstones for improving treatment outcomes in MB patients.
Introduction
Since the first report on the efficacy of craniospinal irradiation (CSI) following surgery in 1969 [1], treatment methods for medulloblastoma (MB) have evolved as a result of improvement in radiotherapeutic and neurosurgical techniques, as well as the development of novel combination chemotherapeutic agents [2,3]. In addition, recent advances in the genomic characterization and molecular understanding of this tumor will also lead to better targeting and individualized treatment of this cancer [4,5].
The traditional strategy for treatment of this tumor has been maximal curative surgical resection, followed by optimal radiation therapy (RT) including CSI and systemic chemotherapy. However, the detailed characteristics of failure patterns after long-term follow-up have not been well reported.
This study was conducted in order to assess our long-term clinical results of multimodal treatment for MB. In particular, we focused on the analyses of failure patterns after treatment and discussed appropriate RT technique based on the study results.
Materials and Methods
1. Study population
Among the 119 MB patients who received RT between January 1992 and October 2009, 106 patients were eligible for inclusion. Patients with initial extraneural metastasis (n=2), patients who were referred from other institutions after recurrence (n=3), patients who were lost to follow-up so that the disease status could not be traced (n=6), and patients who were diagnosed with other histopathology in the pathologic review (n=2) were excluded from this study; the two latter patients were histologically confirmed with atypical teratoid/rhabdoid tumor and anaplastic ependymoma. All eligible patients were histopathologically proven to have MB and received multimodal treatment with a curative aim.
Patient characteristics are summarized in Table 1. The median age was 7 years (range, 0 to 50 years). Cerebrospinal fluid (CSF) examination was performed in a majority of patients (n=97, 91.5%), and 19.8% (n=21) of the cohort showed positive CSF cytology. Whole spinal magnetic resonance imaging (MRI) was performed in 102 patients (96.2%), and spinal seeding metastasis was found in 28.4% (n=29). A total of 85 cases (80.2%) were available for review of pathological subclassification by an experienced neuropathologist. There were 57 classic, 26 desmoplastic/extensive nodular (DM/EN), and two anaplastic/large cell types. CSF spread was more common in classic subtype, compared with DM/EN type (52.6% and 31.6% for M0 and M3, respectively vs. 73.1% and 3.8% for M0 and M3, respectively; p=0.007).
2. Surgery
All patients underwent curative surgical resection by specialized neurosurgeons. Extent of surgical resection was assessed according to the operation findings and postoperative MRI, which was taken within 48 hours after operation. Gross total resection (GTR) was defined as total resection of primary tumor without remnant lesion in both operation finding and postsurgical MRI. Near total resection (NTR) was defined as the state of very small residual disease left in the operation record, but with no significant remnant lesion in postsurgical MRI. GTR/NTR was achieved in 30.8% of cases in the 1990s, increasing to 74.1% in the 2000s (p < 0.001).
In risk stratification, standard-risk group was defined by localized disease (M0), favorable resection status (GTR/NTR), and patients with > 3 years of age. High-risk group was defined as having at least one of the following characteristics: M+ disease, incomplete resection status (subtotal resection, STR), or patients with ≤ 3 years of age.
3. Radiotherapy
A cobalt 60 teletherapy unit (1990s) or 4-6 MV X-rays was used for delivery of RT. CSI was routinely prescribed for all but four patients. Whole brain RT with posterior fossa boost was performed in two adolescent patients due to poor general condition. The two remaining patients ≤ 2 years old received CSI after disease progression, which occurred during pre-RT chemotherapy. The median total dose and fraction size of CSI in 102 patients who had received CSI were 36 Gy (range, 21 to 48.5 Gy) and 1.5 Gy (range, 1.5 to 1.8 Gy), respectively. According to the reduced CSI dose protocol (protocol initiated from September 2003), CSI dose of 36 Gy was reduced to 23.4 Gy in patients with a standard-risk group, and CSI dose of 39-45 Gy was reduced to 23.4-30.6 Gy in patients with ≥ M1 stage.
With the introduction of a computed tomography (CT) simulator in 2000, we applied CSI in the supine position for patients in poor condition [6]. Currently, we are routinely using the supine position for CSI. The median total tumor bed dose and fractional dose were 54 Gy (range, 21 to 60.6 Gy) and 1.8 Gy (range, 1.8 to 2 Gy), respectively. CSF seeding lesions in imaging studies were treated with additional boost RT with a dose range of 5.4 to 10.8 Gy.
RT methods for posterior fossa and tumor bed boost have changed with time, and 48 patients (45.3%) were treated using the 3-dimensional conformal RT (3D CRT) technique. After 2002, posterior fossa fields were reduced to the primary tumor bed plus a margin of 1.5-2 cm (usually standard risk group) or posterior fossa up to 45 Gy, followed by a reduced field to the primary tumor bed plus a margin of 1.5-2 cm. Treatment sequences were usually CSI followed by focal tumor bed boost, but patients with pre-existing bone marrow suppression or poor general condition were treated with tumor bed first followed by CSI. The median time interval between surgery and RT was 4.4 weeks (range, 0.3 to 71.1 weeks). RT was delayed more than 3 months in 12 patients, but the majority of patients in this group (nine patients) were ≤ 3 years of age.
4. Chemotherapy
Combination chemotherapy was administered to 88 patients (83%). In early periods, chemotherapy was not routinely prescribed in standard risk patients. In total, 11 patients with M0, three with M1, one with M2, and three with unknown M stage did not receive chemotherapy.
In patients > 3 years of age, pre-RT chemotherapy of one cycle followed by RT and subsequent chemotherapy was the most preferred treatment sequence. During the RT course, vincristine was administered weekly in most patients. Median seven cycles (range, 1 to 12 cycles) of chemotherapy was administered after RT. Cisplatin and/or cyclophosphamide-based combination chemotherapeutic agents (vincristine/cisplatin/ACNU/prednisolone, vincristine/cisplatin/VP16 and vincristine/cisplatin/CCNU) were most commonly used. From September 2003, four standard-risk MB patients were enrolled in the reduced CSI dose protocol (KSPNO M051). Six high-risk MB patients were enrolled in high-dose chemotherapy, followed by peripheral blood stem cell transplantation (PBSCT) and reduced CSI dose protocol [2]. For patients ≤ 3 years of age, prolonged chemotherapeutic schedule before RT was used routinely in order to delay RT administration.
5. Study end-point and statistical analysis
Statistical analysis was performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL). Overall survival (OS) rates were calculated from the date of surgery. Disease recurrence was defined as relapse from no evidence of disease status after completion of planned treatment including RT. Recurrence, secondary malignancy, and death from any cause were counted as events for calculation of event-free survival (EFS). Recurrence-free interval (RFI) of recurrent cases was calculated from the date of surgery to the date of disease recurrence.
Results
1. Survival and prognostic factors
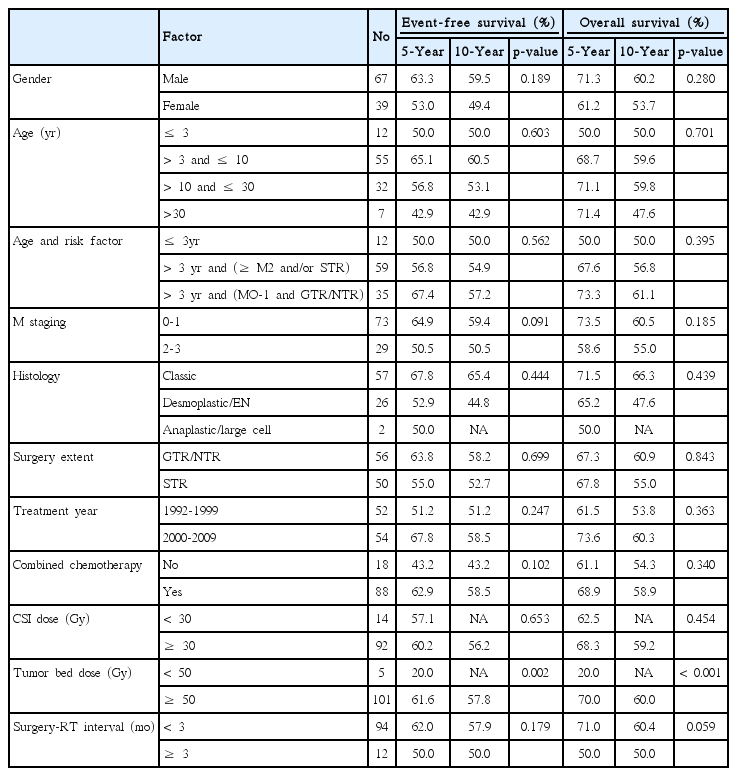
The median follow-up duration was 83 months (range, 3 to 248 months) for the entire population and 132 months (range, 31 to 248 months) for survivors. A total of 43 deaths (40.6%) occurred from the following causes: 28 deaths after recurrence; seven deaths from treatment-related toxicities; three deaths from second malignancies including one case of acute lymphoblastic leukemia and two brain tumors; three deaths from pre-RT disease progression in patients ≤ 2 years of age; and two deaths from unknown reasons. Five- and 10-year OS rates were 67.6% and 57.9%, respectively. Five- and 10-year EFS rates were 59.6% and 55.9%, respectively (Fig. 1). Results of univariate analyses of EFS and OS are shown in Table 2. Sex, age, M stage, and extent of surgical resection did not significantly affect EFS or OS in the entire population.

Kaplan-Meier curves for major study end-points. Overall survival (OS) and event-free survival (EFS) curves in the entire group (A), OS and EFS according to the risk stratification (B, p=0.248; C, p=0.380), and OS according to recurrence (D, p < 0.001).
In a subgroup of 94 patients > 3 years of age, we found that the total tumor bed dose < 54 Gy was administered in two patients and surgery-RT interval was delayed for more than 3 months in three patients, suggesting that most patients had received a definitive curable dose of RT to the tumor bed within the optimal postsurgical interval.
In a subgroup of 12 patients ≤ 3 years of age, the estimated median OS was 41 months (range, 5 to 227 months). In five patients between 2 and 3 years of age, only one patient with M3 died of disease. The four remaining patients were still alive without evidence of relapse at the time of analysis (survival duration, 146 to 228 months). Among these five patients, four received prolonged pre-RT chemotherapy until the age of 3 years old before RT. One remaining high-risk patient was enrolled in the KSPNO M051 protocol and received high-dose chemotherapy followed by PBSCT before RT. In contrast to the outcomes in patients between 2 and 3 years of age, treatment outcomes were poor in the seven patients ≤ 2 years old, with 5-year OS rates of only 28.6% and five deaths. Three patients died due to disease progression during postsurgical chemotherapy despite administration of RT after progression. In the two remaining patients, the causes of death were chemotherapy-induced toxicity. Two patients diagnosed in 2005-2006 and treated with GTR, chemotherapy, and a curable dose of RT (CSI ≥ 30 Gy and tumor bed dose ≥ 54 Gy) were still alive without disease at 61 and 70 months after diagnosis, respectively. The ages of these two patients at the time of diagnosis and RT administration were 21 and 36 months, and 20 and 37 months, respectively.
2. Initial patterns of failure
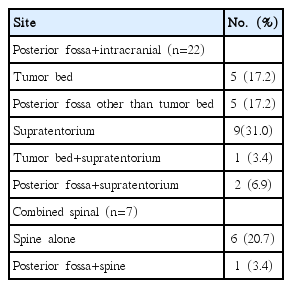
A total of 29 recurrences were noted (crude rate, 27.4%). The results of initial patterns of failure are summarized in Table 3 and representative recurrent cases are shown in Fig. 2. Among 12 supratentorial recurrences (with or without other site metastasis), five recurrent cases occurred in the subfrontal areas. All five patients with subfrontal recurrences initially received 3D CRT using a CT simulator. Among 31 supine-positioned patients, three recurred from supine position, and among 71 prone-positioned patients, two recurred from prone position. Other locations of supratentorial recurrence were the third ventricle, periventricular areas, basal ganglia, parietal lobe, and fronto-temporal lobe. There were no cases of recurrence in patients with M1 stage.
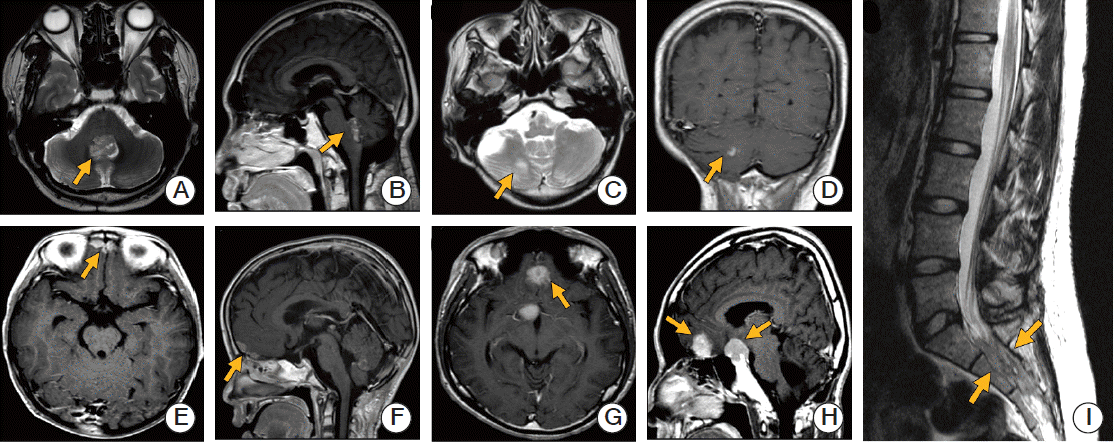
Illustrations showing representative recurrent cases. Primary tumor bed recurrence (A, B), posterior fossa recurrence outside tumor bed (C, D), supratentorial (subfrontal) recurrence (E, F), supratentorial (subfrontal and suprasellar) recurrence (G, H), and sacral recurrence (I) are shown.
Boost field reduction from the posterior fossa to the tumor bed only did not increase the relapse rate in the posterior fossa area. Posterior fossa recurrences (including tumor bed and combined spinal recurrences) occurred in 11 (18.3%) out of 60 patients who received the entire posterior fossa boost, and three (6.5%) out of 46 patients who received the tumor bed boost only (p=0.298). Among three recurrences developed from the tumor bed boost only, two occurred in the tumor bed and one in the posterior fossa other than the tumor bed.
Primary tumor bed/posterior fossa recurrences were more common in patients treated with STR (11/50 [22%] in STR vs. 2/56 [3.6%] in GTR; p=0.016). However, other site (other intracranial/spinal) recurrences were more common in patients treated with GTR (11/56 in GTR vs. 5/50 in STR; p=0.016). Total resection rate was lower in DM/EN subtypes and all recurrences occurred in the primary tumor bed/posterior fossa without spinal relapse (GTR rate: 42.3% in DM/EN vs. 66.7% in classical subtype; p=0.015).
RT methods were marginally associated with the recurrence pattern. Overall recurrences were more common in the 2D era (20/58 in 2D vs. 9/48 in 3D; p=0.08). Among 20 recurrences treated with 2D RT, 11 recurrences developed in the posterior fossa and five in the spinal area. Among nine recurrences treated with 3D RT, two recurrences developed in the posterior fossa and two in the spinal area.
Among seven patients with spinal relapses, four patients with M0 received 0, 30, 36, and 36 Gy of CSI, and three patients with M3 received 36, 39, and 39 Gy of CSI.
3. Disease recurrences, salvage treatment and occurrence of second malignancies
The median RFI after initial surgery was 22 months (range, 3 to 84 months) and the median postrecurrence survival was 11 months (range, 0 to 192 months). Recurrent sites did not significantly affect OS or postrecurrence survival.
Salvage treatment modalities were selected according to the patients’ performance status, disease extent, and the physicians’ preferences. Reirradiation with a dose range of 19.5 to 41.4 Gy was administered in 12 patients with or without other treatments, and gamma-knife radiosurgery was performed in one patient. The detailed characteristics of 12 reirradiation cases are shown in Table 4. Salvage surgery was preferentially performed in the posterior fossa or other solitary intracranial recurrences. Spinal seeding metastasis was mainly managed by chemotherapy and/or focal reirradiation.
Long-lasting survival after successful salvage could be achieved in only five patients. In these five patients, three patients were managed with reirradiation with/without chemotherapy, one with combination chemotherapy alone, and the remaining one with salvage surgery, high-dose chemotherapy, and autologous stem cell transplantation. Four patients were still alive without evidence of disease after 192, 143, 41, and 24 months postrecurrence. The remaining patient died after 70 months postrecurrence due to late second relapse. One patient with initial M0 stage experienced recurrence at S2-4 levels of subarachnoid space with sacral bony extension (Fig. 2I). We found that the sacral CSI field was insufficient, and salvage partial laminectomy, chemotherapy, and reirradiation of 36 Gy to L5-sacrum were performed. The patient continued to live without evidence of disease for 41 months after recurrence.
Pathologically confirmed second brain tumors developed in three patients with the following characteristics: atypical meningioma at the anterior interhemispheric fissure 201 months after RT, low-grade astrocytoma at the left cerebellum 124 months after RT, and anaplastic astrocytoma at the pons 93 months after RT. The patient with meningioma was alive at the time of analysis and the two remaining patients died from the second tumors.
Discussion
Because our patients had received fairly consistent multimodal treatment, we could eliminate the impact of RT doses and volume on outcomes and could assess the appropriateness of the CSI technique.
Analyses of the recurrence pattern showed that there is room for improvement in outcomes by improving surgery and RT techniques. The recurrence rate of primary tumor bed (20.6%) was relatively low, reflecting the appropriateness of surgery and RT administration to the tumor bed. However, the frequency of primary tumor bed/posterior fossa recurrence was still higher in cohorts treated by STR. Reduced boost volume confined to the tumor bed rather than the entire posterior fossa did not increase posterior fossa relapse. We found a high frequency (50% of supratentorial relapses) of subfrontal recurrence and a sacral relapse after a tight sacral CSI field. Subfrontal areas near cribriform plates are potentially high-risk regions of underdose, requiring special concerns in the RT field design [7,8]. Excessive eye-sparing RT technique or unstable prone CSI position might increase the risk of relapse in these areas. In our study, all five subfrontal recurrences developed in the 3D era and excessive eye-block might have caused the recurrences. Another hypothesis suggested by Sun et al. [7] is that lying in the prone position during the operation could facilitate the migration of tumor cells to the cribriform plate due to the gravitational effect, which might cause subsequent tumor recurrence. Given the low successful salvage rate of recurrence and surgically restorable features of cataracts, proper coverage of the cribriform plate is warranted. In the modern era, with the introduction of CT simulation to define accurate target volumes and more stable supine patient position to maintain consistent set-up, we expect that recurrence rates will be reduced.
With changes in time, we observed a gradual improvement in survival outcomes in terms of 5-year OS rates of 61.5% in 1990 to 73.6% in 2000, which might be attributed to several factors related to the development in surgical, radiotherapeutic, and chemotherapeutic approaches. The treatment outcome in 2000 was comparable with that of a recent report [9]. Recently, reduced-dose CSI with chemotherapy and refined tumor bed boost for sparing critical organs with 3D CRT or intensity-modulated radiation therapy (IMRT) techniques have resulted in reduced toxicity without a detrimental effect on local control [3].
The surgical extent, age, M stage, or RT doses that were considered important for survival outcomes in the prior studies were not statistically significant prognostic factors in our study, which is suspected due to the small sample size or tailored treatment. Patients with M1-M3 received higher radiation doses than patients with M0. GTR/NTR resulted in high posterior fossa control, but did not affect survival due to the high rate of distant relapses. More curable control of a primary site might change the patterns of disease relapse and increase distant recurrences [10]. Paulino et al. [3] reported that distant spinal subarachnoid space recurrences were the predominant patterns of failure in the high-dose IMRT era. Young age, although not statistically significant, affected the prognosis. In patients ≤ 2 years old, the main contributing factors for deteriorated OS in early periods were poor tolerance to chemotherapy and delayed or reduced doses of RT. Radical surgery and curable doses of RT delivery could not usually be given and disease progression during pre-RT chemotherapy or treatment-related toxicity were the main reasons for deaths in the 1990s. However, better outcomes could be achieved after 2000. Our study also showed that the prognosis of patients between 2 and 3 years of age could be comparable to that of those > 3 years old, if managed properly.
Based on the results of several studies, a reduced CSI dose of 23.4 Gy and chemotherapy is currently the preferred treatment for standard-risk MB [11,12]. Clinical trials with reduced-dose CSI and intensified chemotherapy are currently ongoing for high-risk MB patients [2]. In our study, in high-risk MB patients among 10 (four standard-risk and six high-risk) patients enrolled in the reduced-dose CSI protocol, there was only one case of disease recurrence and death. This finding is promising, but longer follow-up study is required.
The prognostic significance of histological subtype has been controversial [13,14]. DM/EN subtypes showed low CSF seeding rate; however, complete resection rate was also low, which resulted in a high rate of primary site recurrences. A previous study [15] pointed out that complete resection of DM histology could be challenging because of the lateralized location of the tumor, but that the differences in prognosis between subtypes could be eliminated with improvement of surgical techniques in the modern era. Our study also found no statistical difference between the subtypes in terms of OS.
OS after disease recurrence was significantly deteriorated and successful salvage was achieved in only five patients despite the various efforts of salvage treatments. Among 12 reirradiation cases, there were three successful salvage and long-term survivors. Bakst et al. [16] reported successful and promising treatment outcomes after reirradiation with median postrecurrence survival of 45 months. They found that reirradiation was most beneficial to patients with no evidence of disease after surgical re-resection and suggested trimodality therapy as a promising option in recurrent MB.
Conclusion
In conclusion, in our long-term institutional analysis of MB patients, integration of multimodal treatment including maximal surgical resection and proper CSI resulted in a gradual increase in survival. Our patterns of failure analyses demonstrated that there is room for improvement in outcomes with more sophisticated and optimal RT techniques (complete coverage of craniospinal subarachnoid space, tailored RT dose according to M stage and reduced boost volume to the tumor bed only rather than the entire posterior fossa). The results in our series are encouraging and we hope to cure this devastating pediatric tumor in the near future with improved understanding of the disease and more advanced technological approaches.
Notes
Conflict of interest relevant to this article was not reported.