The Impact of Molecularly Targeted Treatment on Direct Medical Costs in Patients with Advanced Non-small Cell Lung Cancer
Article information
Abstract
Purpose
To investigate the impact of targeted treatment on direct medical costs of patients with advanced non-small cell lung cancer (NSCLC).
Materials and Methods
Medical records of 108 stage IIIB/IV NSCLC patients treated in Seoul National University Hospital between 2003 and 2009, were reviewed to collect medical resources utilization data from the diagnosis of stage IIIB/IV NSCLC to the end of active anti-cancer treatment. The direct medical costs were calculated by multiplying the number of medical resources used by the unit price. All costs were expressed in US dollars for each patient.
Results
The mean total direct medical costs were $34,732 (standard deviation, 21,168) in the study cohort. The mean total direct medical costs were higher in epidermal growth factor receptor (EGFR) mutation (EGFR MT)–positive patients than EGFR wild-type (EGFR WT) patients ($41,403 vs. $30,146, p=0.005). However, the mean monthly direct medical costs did not differ significantly between EGFR MT–positive patients and EGFR WT patients ($2,120 vs. $2,702, p=0.119) because of the longer duration of active anti-cancer treatment in EGFR MT–positive patients. This discrepancy was mainly attributable to EGFR MT–positive patients’ lower non-chemotherapy costs ($948 vs. $1,522, p=0.007). The total and monthly direct medical costs of ALK fusion–positive patients who did not receive ALK inhibitors did not differ from WT/WT patients.
Conclusion
This study suggests that the availability of targeted agents for EGFR MT–positive patients lowers the mean monthly medical costs by prolonging survival and diminishing the use of other medical resources, despite the considerable drug costs.
Introduction
Lung cancer is the third most common cancer in the world and is responsible for the highest cancer mortality rate [1]. Non-small cell lung cancer (NSCLC), comprising 85% of total lung cancer cases, is a devastating disease with only a 10% 5-year survival rate in the advanced stage [2]. The significant economic burden of lung cancer is well documented. In 2004, the costs of lung cancer care in the United States were estimated at approximately 20% of total medicare expenditures on cancer care, greater than the total costs of colorectal and prostate cancer [3].
The introduction of molecular biomarkers and targeted agents dramatically improved treatment outcomes of advanced NSCLC [4]. Administration of gefitinib (Iressa, AstraZeneca) and erlotinib (Tarceva, Hoffman-La Roche) resulted in prolonged progression-free survival (PFS) of stage IV NSCLC patients harboring drug-sensitive epidermal growth factor receptor (EGFR) mutation (MT) [5-8]. Crizotinib (Xalkori, Pfizer) recently demonstrated 4.7-month prolongation of PFS as a second-line agent in patients with NSCLC harboring ALK fusion, in a phase III randomized controlled trial [9]. These clinical trials improved quality of life, mainly by reduced side effects; there were more grade 3 or 4 adverse events in the chemotherapy group [6-8]. Broad application of these agents, however, has been impeded by expensive biomarker testing and drug costs. Although many studies investigated the economics of introducing targeted agents to treat unselected NSCLC populations, differences in medical cost among relevant molecular subgroups has not been thoroughly investigated [10-14].
To elucidate the economics of molecular targeted therapy in advanced NSCLC patients, we investigated the direct medical costs of stage IIIB/IV NSCLC patients treated at Seoul National University Hospital, from 2003 to 2009. During this period, EGFR tyrosine kinase inhibitor (TKI) was available but crizotinib was not (this drug was introduced in a phase I trial in 2008). By contrasting the economic burden of patients with EGFR MT, ALK fusion and both wild-types (WT/WT), we could estimate the possible impact of molecularly targeted treatment on direct medical costs of advanced NSCLC.
Materials and Methods
1. Study population
The population of this study includes the subjects from the final analysis of the previous study [15]. From the parent population of 1,166 patients with advanced, non-squamous NSCLC managed at Seoul National University Hospital between 2003 and 2009, 23 patients were diagnosed as ALK fusion–positive. Each ALK fusion–positive patient was identically matched to two EGFR MT–positive (n=46), and two WT/WT patients (n=46) by their age at diagnosis, gender, stage of cancer, and smoking status. From the final cohort comprising 115 subjects, seven patients who did not receive any active anti-cancer treatment were excluded. Forty-four EGFR MT–positive, 22 ALK fusion–positive, and 42 WT/WT patients were included in this analysis. Clinical and pathologic information, including histology and molecular subtypes, was utilized from the dataset of the previous study. Reason for termination of active anti-cancer treatment was abstracted as well.
2. Data collection and quantification of medical resource utilization
An oncologist (J.-K.L.) reviewed electronic medical records of individual patients to determine which items were related to cancer treatment. The items assessed for cost included hospital visits, surgery, chemotherapy, radiotherapy, diagnostic or laboratory studies, and other adjunct treatments. The details of each item are summarized in Table 1.
Investigators collected and captured individual data on medical resource utilization. Another oncologist (D.-W.K.) did a final validation after the entire data collection process.
The direct medical costs were calculated by multiplying the number of medical resources used by the unit price of each. The unit price of each medical resource was obtained from the National Health Insurance in Korea, based on the standard prices of 2011. For items not covered by the National Health Insurance, the actual costs paid by the patients were obtained from Seoul National University Hospital.
The total direct medical costs were analyzed throughout the duration of active anti-cancer treatment, a period defined between the date of stage IIIB/IV diagnosis and the end of active anti-cancer treatment. Active anti-cancer treatment was defined as surgery, chemotherapy or radiotherapy. Individual patients had different survival durations depending on molecular subtypes [15]. We assumed that the study population had different active anti-cancer treatment durations that led to different medical resource utilization. To adjust the total direct medical costs with active treatment duration, the mean monthly direct medical costs were calculated by dividing total direct medical costs by the active treatment duration. All costs are presented in 2012 US dollars (October 2012); this exchange rate approximately corresponds to the value of average exchange rate of US dollars during the past 10 years (1 USD=1,100 KRW).
3. Statistical analysis
Descriptive analyses were performed to clarify the distributions of demographic, clinical characteristics and medical costs. The difference between the mean total direct medical costs by patients’ characteristics and total and monthly direct medical costs by molecular subtypes were determined by t tests or ANOVA with log transformation of the costs. When comparing the chemotherapy and non-chemotherapy costs between each molecular subgroup, the Kruskal-Wallis test was used because the distribution is right-skewed. Direct medical cost of each molecular subgroup was analyzed over time, at 6-month intervals until 1 year from the start of active treatment, and 12-month intervals thereafter. Data analyses were performed using SPSS ver. 20.0 (SPSS Inc. Chicago, IL).
4. Ethical considerations
The Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-1104-109-359) reviewed and approved the study protocol, and exempted the study from the obligation to obtain informed consent. This study was performed in observance of the World Medical Association’s Declaration of Helsinki.
Results
1. Patient characteristics
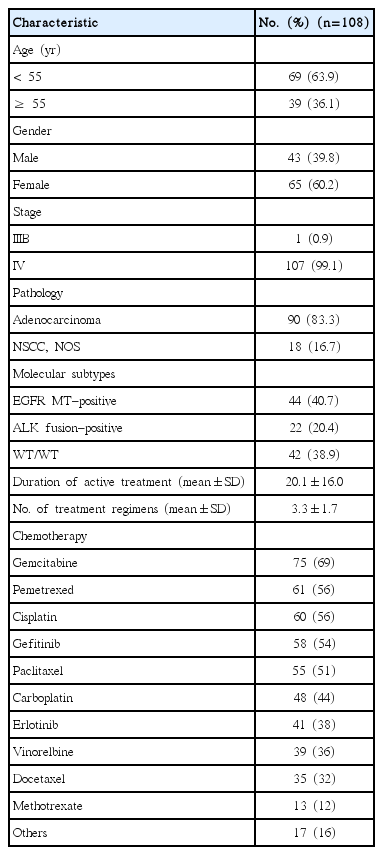
One hundred and eight patients with stage IIIB/IV non-squamous NSCLC were included in this analysis. The median age was 51, with over 60% of patients being female, and over 80% having adenocarcinoma histology. The mean duration of active anti-cancer treatment in the study population was 20.1 months and the average number of treatment regimens was 3.3 (standard deviation, 1.7). The most common chemotherapy treatments were gemcitabine (69% of patients), pemetrexed (56%), cisplatin (56%), and gefitinib (54%) (Table 2). The most common reason for termination of active anti-cancer treatment was disease progression (57.4%), followed by patient refusal (19.4%), and adverse events or functional decline (14.8%).
2. Total direct medical costs
The mean total direct medical cost was $34,732. Chemotherapy costs comprised the largest portion (61.2%) of the direct medical costs, followed by diagnostic studies and adjunct medication (Fig. 1). The mean total direct costs were higher in patients under 55 than over 55 ($36,006 vs. $32,480), male than female ($40,446 vs. $30,953, p=0.004), stage IV than IIIB ($34,920 vs. $14,660; p-value, not acquired) and adenocarcinoma than non-small cell carcinoma, not otherwise specified ($35,770 vs. $29,545) (Table 3).
3. Direct medical costs by molecular subtypes
Out of 108 patients, 22 were ALK fusion–positive, 44 were EGFR MT and 42 were WT/WT. The mean duration of active anti-cancer treatment was 23.7 months in EGFR MT–positive, significantly longer than in ALK fusion–positive and WT/WT patients (p=0.011) (Table 4). Among the EGFR MT– positive patients, 42 (95.4%) received EGFR TKIs but none of the ALK fusion–positive patients received ALK inhibitors.
Total direct medical costs were significantly higher in EGFR MT–positive patients compared to the other two groups (p=0.003) (Table 4). Monthly direct medical costs, however, did not differ across the groups (p=0.294); non-chemotherapy costs in EGFR MT–positive patients were lower than the other two groups (p=0.026) although chemotherapy costs did not differ (p=0.234) among the three groups (Table 4).
The differences were highlighted when comparing EGFR MT–positive patients, who were treated using targeted therapies, and EGFRWT patients (including ALK fusion–positive and WT/WT patients), who were treated using conventional therapies. Total direct medical costs were significantly higher in EGFR MT–positive patients compared to EGFR WT patients ($41,403 vs. $30,146, p=0.005). However, monthly direct medical costs between EGFR MT–positive patients and EGFR WT patients did not significantly differ ($2,120 vs. $2,702, p=0.119).
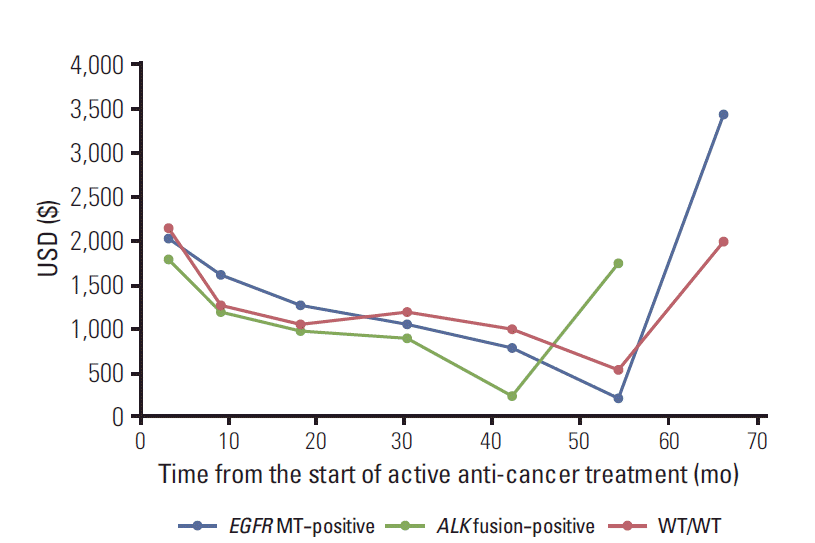
For patients with ALK fusion, both the total direct medical costs ($22,463) and the monthly direct medical costs ($2,397) did not differ significantly compared to WT/WT patients (vs. $34,171 and $2,862, p=0.070 and p=0.656, respectively). Monthly direct medical cost and their components over time are plotted in Fig. 2. In all three groups, total medical cost was the highest at the start of the active treatment, gradually decreased, and increased again approaching termination of active treatment.
Discussion
Tumor histopathology has long been a standard parameter for treatment selection for NSCLC patients. The efficacy of various chemotherapeutic agents used in the treatment of NSCLC was similar among the different histologic subtypes [16]. The treatment of NSCLC has evolved substantially with the advances of therapy specifically targeting molecular biomarkers. Several clinical trials find that targeted therapy enhances therapeutic efficacy, reduces toxicity and leads to an improved quality of life [6-8], but with increased drug and diagnosis costs. Through this study, we show the potential value of molecularly targeted treatment on the reduction of economic burdens in patients with advanced non-small cell lung cancer.
EGFR MT–positive patients’ total direct medical costs were the highest among the three groups. When considering treatment duration, the monthly costs of EGFR MT–positive patients were significantly lower. The difference was mainly due to their low non-chemotherapy costs. This suggests that the availability of targeted treatment for EGFR MT–positive patients lowers the mean monthly medical costs by prolongation of survival and reducing use of other medical resources, despite the considerable drug cost and cost for EGFR mutation analysis ($164 in Seoul National University Hospital, direct sequencing of EGFR exon 18-21). The monthly costs of adjunct medication were significantly lower in EGFR MT–positive group ($211 vs. $287, p=0.008) similar to the results of the INTEREST trial [17]. This can be partly explained by superior safety outcomes.
ALK fusion–positive patients’ total and monthly direct medical costs did not differ from those of WT/WT patients. The ALK inhibitor was not available for the ALK fusion– positive patients in this study. They received the same conventional anti-cancer treatment as WT/WT patients, resulting in no difference in the direct medical costs. After 2008, a significant portion of advanced NSCLC patients harboring ALK fusion received crizotinib. Targeted treatment may be economically preferable for these patients for the same reason. Further economic analysis can provide additional insight into the impact of targeted treatment on medical costs.
In this study, the medical cost for male patients was significantly higher. We could not find the reason for this difference in our analyses. Findings are inconsistent on medical cost difference by gender in lung cancer patients [18]. Female lung cancer patients were more likely to be never-smokers [19], and tended to be less symptomatic [20] compared with male patients with lung cancer. This may partly explain the gender difference in direct medical cost in our study.
There are several limitations to this study. First, the characteristics of this study population reflect the characteristics of ALK fusion–positive patients, not regular NSCLC population due to the sampling methods used. Second, medical costs were only collected during the active treatment period but not throughout the entire survival period of patients. Medical cost at the end of life could not be collected largely because of referral to hospice/palliative care facilities or loss. Medical cost in the final month is disproportionally high because of life-sustaining care [21]. Medical resource use in the end-of-life is closely related to end-of-life discussions between patient, caregiver, and physician [22], another factor determining the direct medical cost of advanced NSCLC patients. Third, adjunct medication data was collected only from prescribed medications but not itemized by the side effects that were treated. This study, therefore, should not be generalized to all NSCLC cases. Last, monthly-basis cost analysis has limited value especially for the aspect of health care system, although this analysis provided information on the medical cost per unit time.
Despite these limitations, the study contributes valuable information to the economic characterization of NSCLC treatment. While previous studies examined the economics of molecular targeted therapies in general NSCLC populations [10-14], this is the first study investigating the difference of direct medical cost among molecular subgroups of NSCLC. The results pinpointed the impact of targeted therapies on a potential reduction of medical resource utilization and associated cost, while precisely collecting cost data from individual patients and individually selected items, under an oncologist’s recommendation. The study results reflect standard clinical practice and will result in a better refinement of treatment selection parameters.
Conclusion
This study quantified medical resource utilization and associated costs for treatment of advanced NSCLC compared across molecular subgroups. EGFR MT–positive patients had a definite molecular target and received appropriately targeted agents. Their monthly medical costs were the lowest among the three groups, by reducing use of other medical resources despite high drug costs. However, ALK fusion– positive patients did not have available targeted agents and their monthly medical costs were no different from WT/WT patients. In conclusion, molecular targeted therapy can lower monthly direct medical cost by diminishing the use of medical resources other than chemotherapy, which results from their better clinical outcomes.
Notes
Dong-Wan Kim has acted as a consultant and has received honorarium from Pfizer. Se-Hoon Lee has acted as a consultant and has received honorarium from Pfizer. Young-Joo Kim is an employee of Pfizer.
Acknowledgements
This study was sponsored in part by Pfizer Pharmaceuticals Korea Limited.