Breast Cancer Metastasis to the Stomach Resembling Early Gastric Cancer
Article information
Abstract
Breast cancer metastases to the stomach are infrequent, with an estimated incidence rate of approximately 0.3%. Gastric metastases usually are derived from lobular rather than from ductal breast cancer. The most frequent type of a breast cancer metastasis as seen on endoscopy to the stomach is linitis plastica; features of a metastatic lesion that resemble early gastric cancer (EGC) are extremely rare. In this report, we present a case of a breast cancer metastasis to the stomach from an infiltrating ductal carcinoma (IDC) of the breast in a 48-year-old woman. The patient had undergone a left modified radical mastectomy with axillary dissection nine years prior. A gastric endoscopy performed for evaluation of nausea and anorexia showed the presence of a slightly elevated mucosal lesion in the cardia, suggestive of a type IIa EGC. A histological examination revealed nests of a carcinoma in the subepithelial lymphatics, and immunohistochemical staining for estrogen receptor was positive. This is an extremely rare case with features of type IIa EGC, but the lesion was finally identified as a cancer metastasis to the cardia of the stomach from an IDC of the breast.
INTRODUCTION
Breast cancer is the most common cancer of females in South Korea and approximately 50% of patients will develop a distant metastasis during their lifetime. Instead of isolated local recurrence, disseminated metastatic disease is more common with relapse of the disease, while fewer than ten percent of women present with metastatic disease at the time of diagnosis. The most commonly associated sites of a distant tumor metastasis are the bone, liver, lung, soft tissue and adrenal glands (1).
Breast cancer metastases to the stomach are infrequent, and the estimated incidence rate of a metastasis is approximately 0.3% (2,3). Gastric metastases are usually derived from a lobular rather than from a ductal breast cancer (3). The most frequent type of a breast cancer metastasis to the stomach as seen on endoscopy is linitis plastica; features of a metastatic lesion that resemble an early gastric cancer (EGC) are extremely rare (3-6).
In this report, I present a case of a lesion with features of a type IIa EGC that was ultimately identified as a metastasis to the cardia of stomach from an infiltrating ductal carcinoma (IDC) of the breast.
CASE REPORT
A 48-year-old woman was admitted with nausea and anorexia. In May 1999, the patient had been diagnosed with breast cancer, IDC stage IIA (pT1cN1M0). Expression of estrogen and progesterone receptor and Her2/neu were all positive. A modified radical mastectomy and axillary lymph node dissection were performed on the left side followed by the administration of adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil for six months. Following chemotherapy, the patient received 20 mg tamoxifen daily and follow-up examinations were conducted regularly.
The patient was disease free until she experienced a frequent cough in February 2007. Pleural effusion was seen a chest X-ray film and a pleural biopsy demonstrated the presence of a metastatic adenocarcinoma. The use of combination chemotherapy was recommended, but the patient refused treatment and only complementary therapies were given.
In May 2007, the patient visited the Integrative Cancer Center, Kyung Hee University for a second opinion. A CT scan showed pleural effusion in both sides of the thorax with a possible lymphangitic metastasis. Lymph node enlargement in the mesenteric root and aortocaval area were noted. Multiple osteoblastic or osteolytic lesions were also noted in the thoracic and lumbar spine and the pelvis. The serum CEA level and CA15.3 level were 6.1 ng/ml and 282.9 U/ml, respectively. In June 2007, combination chemotherapy with cyclophosphamide, doxorubicin and 5-fluorouracil was started, and eight courses of therapy were given. The CEA and CA15.3 levels were markedly decreased to 1.9 ng/ml and 36.42 U/ml, respectively, at the end of the treatment. After chemotherapy, the patient again received 20 mg tamoxifen daily.
In June 2008, the CEA and CA15.3 levels increased to 3.5 ng/ml and 168 U/ml, respectively. A CT scan showed the presence of multiple small-sized hypodense lesions in both hepatic lobes and a moderate amount of pleural effusion on both sides, dilatation of both renal pelvocalyces and both ureters, and an approximate 5.2×2.8 cm-sized soft tissue mass in the right pelvic cavity, suggestive of a Krukenberg tumor. A PET/CT scan showed the presence of disseminated multiple bone metastases with low metabolic activity, including a liver metastasis in the right posterior segment and a liver metastasis in the left medial segment, a malignancy in the right pelvis and a hypermetabolic lesion in the stomach. A gastric endoscopy for evaluation of nausea and anorexia showed the presence of a superficial elevated mucosal lesion (Fig. 1A). Atrophic gastritis was also observed throughout the gastric surface and no other abnormal findings were detected in the esophagus and duodenum. The initial endoscopic impression was a type IIa EGC, and a biopsy was performed. Nests of carcinoma in subepithelial lymphatics were seen. The malignant cells were not specific to breast cancer. The use of immunohistochemical staining determined the status of cellular markers as estrogen receptor (+), progesterone receptor (-), C-erbB-2 (score 1) and GCDFP-15 (+) for luminal secretion of the tumor glands, features that favor the presence of a primary breast tumor (Fig. 2). Palliative chemotherapy with paclitaxel was commenced.
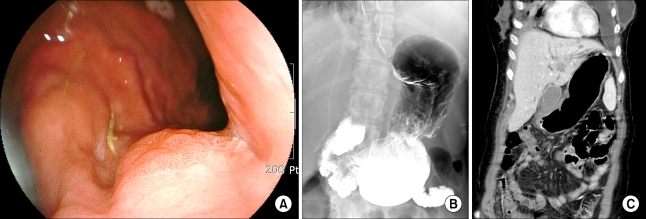
(A) A gastric endoscopic examination reveals a slightly elevated lesion in the cardia, suggestive of type IIa early gastric caner. (B) An upper gastrointestinal series shows multiple nodular filling defects at the lesser curvature side of the gastric cardia, suggestive of a suspicious early gastric cancer. Gastric motility is not impaired. (C) A coronal view of an abdominal CT scan shows multiple liver metastases. Gastric wall thickening is not seen.
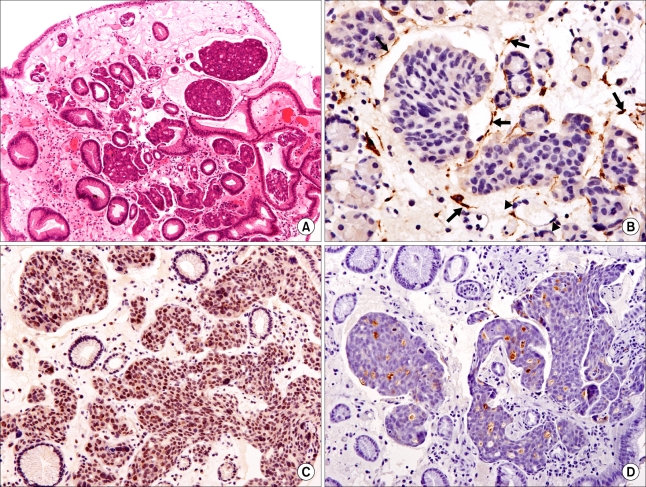
(A) Nests of carcinoma in subepithelial lymphatics are seen (H&E staining, ×100). (B) D2-40 immunostaining is positive for the lymphatic endothelium (arrow) and negative for the capillary endothelium (arrowhead) (Polymer method, ×400) (C) Immunohistochemical staining for estrogen receptor shows strong positivity (×200). (D) GCDFP-15 immunostaining shows positivity for luminal secretion of the tumor glands (Polymer method, ×200).
DISCUSSION
Breast cancer metastases to the gastrointestinal (GI) tract are infrequent, and GI metastases are often not considered in daily practice. Reports on GI metastases in the clinical literature are scarce and are mainly limited to case reports or case series. According to a study by Borst and Ingold, only 17 patients (<1%) were found to have a metastasis to the GI tract including the stomach (7). The incidence of a breast cancer metastasis to the stomach has been found to vary according to the study. The postmortem incidence of gastric metastases has been shown to vary from 7.4% to 18% (3), whereas the frequency of detection of gastric metastases during a lifetime was determined as 6% (8). McLemore et al. conducted a retrospective review of records of patients with a pathological diagnosis of metastatic disease secondary to breast cancer. In this study, 41 of 12,001 patients (with an estimated incidence rate of approximately 0.3%) were found to have metastatic disease to the GI tract. It was also shown in this study that a metastasis to stomach accounted for approximately 28% of GI metastases (2). In a report by Taal et al., gastric metastases were found in 51 of approximately 16,000 endoscopies (with an estimated incidence of approximately 0.3%) performed in patients with a breast carcinoma (3). In South Korea, a breast cancer metastasis to stomach is very rare and, to the best of my knowledge, only nine cases have been reported (5,9-11).
The median interval from diagnosis of breast cancer to the detection of a gastric metastasis has been reported as four to five years (1,3). In South Korea, including the present case, the interval was variable, from 28.3 months to 17 years (5,9-11).
Three pathways are possibly involved in the metastatic spread of a breast carcinoma to the stomach: hematogenous dissemination, lymphatic spread and direct tumor invasion (3). In this case, an endoscopic biopsy showed nests of breast cancer cells in the subepithelial lymphatics without gastric wall infiltration by the tumor (Fig. 2A, C and D). In most of the previous cases, lymphangitic carcinomatosis occurred due to initial hematogenous spread of a tumor to the stomach, with subsequent invasion through the vessel wall into the lymphatics. After hematogenous dissemination to the stomach, the tumor cells fail to traverse the capillary network and implant in the submucosa (12), which may result in the identification of a false negative by a superficial biopsy, as was shown in the present case.
The pathological types of metastatic involvement of the stomach can be categorized into four types-microscopic infiltration, a gross nodule, a gross ulceration and gross hypertrophic wall (13). In this case, a filling defect with or without ulceration was not shown, and rigidity or sclerotic gastric wall thickening was not identified by an abdominal CT scan or upper gastrointestinal tract radiological examination, suggesting that full thickness infiltration by tumor cells, a feature of linitis plastica, was unlikely (Fig. 1B and C). Microscopic infiltration is characterized by a grossly normal appearance and by the presence of foci of cancer cells in the mucosa, submucosa or muscularis propria, and this is presumed to be the most probable type of lesion in the present case (13).
In a report by Taal et al., gastric metastases had been preceded by metastases seen in the bone, skin, lung, colorectal area and other tissues (1). In the present case, metastases to the lung, liver, and bone could be suspected as a source of the gastric metastasis. In a study of autopsy records based on the characterization of 647 primary breast cancers, the lung, liver, and bone were found to have a metastatic frequency of 0.66, 0.61 and 0.70, respectively, and the sites were considered as potential sources of further metastatic dissemination. For the stomach, none of the frequency was significant, and the investigators did not establish any route of dissemination of the breast cancer to the stomach (14).
Cancer cells in the gastric wall are destined to drain into the lymphatics. The diagnosis of lymphatic vascular invasion is made based on the presence of tumor emboli within vascular channels or in open spaces lined by a single layer of endothelial cells. However, the identification of a lymphatic vessel is sometimes impossible in slides stained with hematoxylin and eosin. The use of D2-40 staining, which has been recently available, detects the endothelium of lymphatic vessels but does not detect the endothelium of blood vessels. In the present case, lymphatic vascular invasion was confirmed by the use of D2-40 staining (Fig. 2B). In breast cancers, lymphatic vascular invasion is frequent, and the majority of lymphatics are located in the peripheral (the outer one-third of the tumor) and peritumoral (all of the normal tissue surrounding the tumor) area, an advancing edge of the tumor (15). In the gastric mucosa, the lymphatics are predominantly present in the deeper lamina propria adjacent to the muscularis mucosae, and the submucosal lymphatic channels communicate with intramuscular and subserosal lymphatics. Lymphatic drainage from the cardia is into the left gastric nodes, and the effluents proceed to the celiac lymph nodes. Unfortunately, little is known about the lymphatic pathways in cancer patients as compared with healthy subjects.
Although less common, direct infiltration can occur due to contiguous lymphadenopathy or can occur due to the presence of an adjacent metastatic cancer (14). In the present case, the contiguous node was not enlarged and this finding lead to the possibility of the presence of a gastric metastasis due to contiguous lymphadenopathy as being less likely. In the present case, an abdominal CT scan and PET/CT scan showed no evidence of direct invasion by the neighboring metastatic sites including the liver and the imaging modalities showed no radiological sign of serosal involvement. A limitation of this report is the lack of a necropsy, which might identity the most probable pathway of the breast cancer metastasis to the stomach.
In a report by Taal et al., endoscopy for breast cancer metastasis to the stomach showed three patterns: localized (including a large ulcer and polyp), diffuse infiltration (including linitis) and external compression. The most frequent type was linitis plastica with enlarged, thickened folds and tumor infiltration in the deep layers (3). Therefore, it is not surprising that endoscopic biopsies may be negative for malignancy in many patients. Rarely, a breast cancer metastasis to stomach may resemble EGC. Hwang et al. reported a case with features of EGC (IIb) that occurred at the lower body (5). Dumoulin et al. also reported seven superficial type IIa polypoid lesions (4), and Pera et al. reported a lesion with features of an EGC, diffuse type (6). In the present case, the initial diagnosis was an EGC, IIa. The histological pattern of all of the lesions was an invasive lobular carcinoma (ILC) of the breast, except for the present case (4-6).
When compared to an ILC, an IDC was found to metastasize to the GI tract less frequently. According to the findings of McLemore et al., among 12,001 patients who were diagnosed with metastatic disease secondary to breast cancer, 1,516 (12%) patients had an ILC, while 10,334 (82%) patients had an IDC. Of the breast tumors, an ILC represented 64% of the GI metastases and this finding may reflect the characteristic diffuse growth pattern of this tumor (2). In the present case, the patient's initial tumor cell type of the patient was an IDC, which is known to metastasize to GI tract less frequently than an ILC.
According to the report by Taal et al., the most frequent location was diffuse (50%), and it was followed by middle (40%) and proximal (10%) portions of the stomach in cases of an IDC (3). In South Korea, the locations of gastric metastases from two IDC cases were the body and pylorus (9,11). In the present case, the location was the cardia, where a metastasis to the stomach from an IDC is relatively infrequent.
A diagnosis of a breast cancer metastasis to the GI tract is often difficult because of the relatively low prevalence of an isolated GI metastasis and the prolonged disease-free interval before symptoms present due to the metastasis. The benign appearance of the lesions also attributes to the difficulty in diagnosis (4). Furthermore, endoscopic biopsies might be tumor negative in up to 30% of cases as tumor cell infiltration is localized in the deeper layers that often are not accessible to the biopsy forceps (1,3). Metastatic breast cancer may also be misdiagnosed as a GI primary cancer. The signs and symptoms are often nonspecific and may delay diagnostic evaluation. Symptoms of a gastric metastasis may include abdominal pain, bloating, bleeding, bowel obstruction, nausea and vomiting, early satiety, dysphagia, weight loss and anemia or fatigue (1).
This case is of interest for two reasons. First, a breast cancer metastasis with a feature of type IIa EGC, especially in the cardia, is extremely rare. Second, the primary breast cancer was an IDC that is known to metastasize less frequently to the GI tract as compared with an ILC. In patients with a history of breast cancer, gastric endoscopy should be offered in cases of GI symptoms, even when the symptoms are non-specific (3). In addition, attention is needed for patients with an IDC for a possible gastric metastasis, as was seen in this case report.