Predictive Factors for Radiation Pneumonitis in Lung Cancer Treated with Helical Tomotherapy
Article information
Abstract
Purpose
Predictive factors for radiation pneumonitis (RP) after helical tomotherapy (HT) may differ from those after linac-based radiotherapy. In this study, we identified predictive factors for RP in patients with lung cancer treated with HT.
Materials and Methods
We retrospectively analyzed clinical, treatment-related and dosimetric factors from 31 patients with lung cancer treated with HT. RP was graded according to Common Terminology Criteria for Adverse Events version 4.0 and grade ≥2 RP was defined as a RP event. We used Kaplan-Meier methods to compute the actuarial incidence of RP. For univariate and multivariate analysis, the log-rank test and the Cox proportional regression hazard model were used. We generated receiver-operating characteristics (ROC) curves to define the cutoff values for significant parameters.
Results
The median follow-up duration was 6.6 months (range, 1.6 to 38.5 months). The 2-, 4-, and 6-month actuarial RP event rates were 13.2%, 58.5%, and 67.0%, respectively. There was no grade 4 or more RP. Ipsilateral V5, V10, V15, and contralateral V5 were related with RP event on univariate analysis. By multivariate analysis, ipsilateral V10 was factor most strongly associated with RP event. On the ROC curve, the cutoff values of ipsilateral V5, V10, V15, and contralateral V5 were 67.5%, 58.5%, 50.0%, and 55.5%, respectively.
Conclusion
In our study, ipsilateral V5, V10, V15, and contralateral V5 were significant predictive factors for RP after HT.
Introduction
Thoracic radiotherapy (RT) is the standard treatment for patients with unresectable lung cancer. However, thoracic RT is commonly accompanied by development of radiation pneumonitis (RP), with reported incidence rates ranging from 15% to 45% [1-8]. Because RP is a major sequela of thoracic RT that can influence the clinical course of the patients with lung cancer, many investigators have reported predictive factors for RP [3-5,8-10]. However, most of these studies analyzed patient groups treated with three-dimensional conformal RT (3D-CRT) or linac-based intensity-modulated radiotherapy (IMRT).
Helical tomotherapy (HT), one of the newest conformal RT modalities, employs helical IMRT in which a gantry 6-MV linear accelerator rotates continuously through 360° around the patient using tens of thousands of narrow beamlets, and provides an integrated megavoltage computed tomography (CT) unit that permits real-time verification of patient set-up [11]. HT planning has many advantages, including a more conformal dose distribution and decreasing radiation dose to normal structures in lung cancer [12-14]. On the other hand, because of the helical radiation delivery method, low-dose shower is of concern in HT [15]. Therefore, predictive factors for RP after thoracic HT may differ from those after 3D-CRT or linac-based IMRT. However, few studies have reported such factors.
In this study, we identified the predictive factors for RP in lung cancer patients treated with HT.
Materials and Methods
1. Patient population
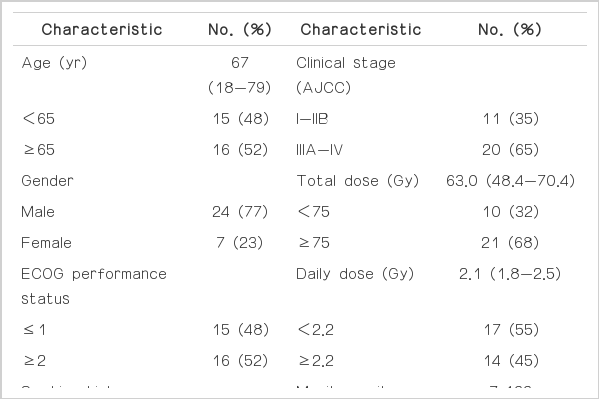
Patient eligibility criteria included: 1) presence of pathologically confirmed inoperable primary lung cancer; 2) receipt of HT with or without chemotherapy; 3) receipt of a total dose of ≥45 Gy; 4) no prior thoracic irradiation; 5) no prior thoracic cancer; 6) no other simultaneous malignancies; 7) available follow-up data. From January 2008 to May 2012, 34 patients with primary lung cancer received HT at our hospital because of advanced tumor stage or medical inoperability. Of those patients, 31 patients met the eligibility criteria and were included in this study.
Each patient had basic laboratory studies, pulmonary function test, chest X-ray, chest CT, magnetic resonance imaging of the brain, and most patients had whole-body positron emission tomography (PET). The clinical TNM stages were determined according to the American Joint Committee on Cancer (AJCC) TNM staging system (7th edition). For all patients, hospital records, laboratory results, and imaging studies were reviewed. Institutional Review Board approval was obtained for the review and analysis of patient data.
2. RT planning
All patients underwent CT simulation in the supine position with arms above their head after immobilization with posterior vacuum bags and anterior vacuum-sealed cover sheets (BodyFix, Medical Intelligence Medizintechnik GmbH, Schwabmünchen, Germany). To reduce movement of the lung by respiration, all patients were asked to take shallow breaths. In all patients, intravenous contrast agents were administered, and axial CT images (3-mm slice thickness) were obtained from above the upper neck through the diaphragm.
The simulation CT data were transferred to a Hi·Art Planning Station (TomoTherapy Incorp., Madison, WI) for inverse planning. The gross tumor volume (GTV) encompassed all detectable tumors and involved lymph nodes determined from chest CT and PET information. Elective nodal irradiation was not done. The clinical target volume (CTV) included the GTV plus 6-8 mm margin [16], and the planning target volume (PTV) was created by adding 8-15 mm margin to the CTV taking into account of target movement by respiration. Normal structures were also delineated. The ipsilateral and contralateral lungs (CLs) were delineated separately to attempt to keep the dose to the CL as low as possible. Other delineated normal structures included spinal cord, heart, and esophagus.
The prescription dose was decided by the physician's own judgment according to tumor size and patients' general condition. A daily dose of 1.8 to 2.5 Gy was delivered at five fractions per week to deliver a total dose of 48.4 to 70.4 Gy. Most prescribed dose fractionation schedules were a total dose of 63 to 66 Gy with daily dose of 2.1 to 2.2 Gy. Of the whole patients, 17 patients (54.8%) were prescribed those fractionation schedules.
Each treatment plan was evaluated with a cumulative dose-volume histogram. In general, plans were considered acceptable if the PTV was covered by 95% of isodose curves, inhomogeneity of the PTV ranged from 95% to 107%, and doses to normal organs were limited in their tolerances. Dose tolerances for normal structures were as follows: total lung (TL) V20<40%, mean lung dose (MLD)<20 Gy, maximum dose to spinal cord<45 Gy, heart V40<50%, and esophagus V55<30%. Planning objectives were prioritized to give the greatest importance to achieving coverage of the PTV and avoiding spinal cord and normal lung, with trying to keep radiation doses in the normal structures as low as possible.
3. Evaluation of RP and statistical analysis
Patients were evaluated with weekly chest X-rays during radiation treatment. Follow-up visits were scheduled at 1 month after completion of RT and every 2-3 months thereafter. Visits were more frequent for those who experienced treatment-related complications. At the time of follow-up visits, basic laboratory studies, chest X-ray, and chest CT scans were taken. When needed, PET was also taken. RP was diagnosed by clinical symptoms along with the characteristic imaging findings, and scored according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.0. RP was assessed in terms of both time to onset and severity. Because RP could initially present during treatment, the time to RP was calculated from the date of RT start. In this study, grade≥2 RP was defined as a RP event.
The Kaplan-Meier method was used to compute the actuarial incidence of RP. The correlation of the development of RP event with potential predictive factors was determined using the log-rank test. Parameters evaluated as potential predictive factors for RP event were: age, gender, the Eastern Cooperative Oncology Group (ECOG) performance status, smoking history, underlying disease, tumor histology, tumor location, AJCC stage, RT total dose, monitor unit, PTV, number of targets, chemotherapy, and several dosimetric parameters (ipsilateral and contralateral MLD, V5-30 in the increment of 5 Gy). For multivariate analysis, the Cox proportional regression hazard model was used. Receiver-operating characteristic (ROC) curves were generated to define the cutoff values for significant parameters. For all analyses, a p-value<0.05 was considered statistically significant. All analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
Patient and tumor characteristics are summarized in Table 1. ECOG performance status was 0 in one patient (3.1%), 1 in 14 patients (45.2%), 2 in 14 patients (45.2%), and 3 in two patients (6.5%). Nine patients (29.0%) had underlying chronic obstructive pulmonary disease, three patients (9.7%) had cerebrovascular disease, and two patients (6.5%) had ischemic heart disease. Eleven patients (35.4%) had squamous cell carcinoma, eight patients (25.8%) had adenocarcinoma, five patients (16.1%) had small cell carcinoma, two patients (6.6%) had neuroendocrine carcinoma, and five patients (16.1%) had non-small cell lung cancer (not confirmed specific histology). AJCC clinical stage was I in five patients (16.1%), II in six patients (19.4%), III in 19 patients (61.3%), and IV in one patient (3.2%). Chemotherapy was implemented in 18 patients: concurrent chemotherapy in 12 patients, induction chemotherapy in three patients, and adjuvant chemotherapy in three patients. The median follow-up period was 6.6 months (range, 1.6 to 38.5 months).
2. Development of RP
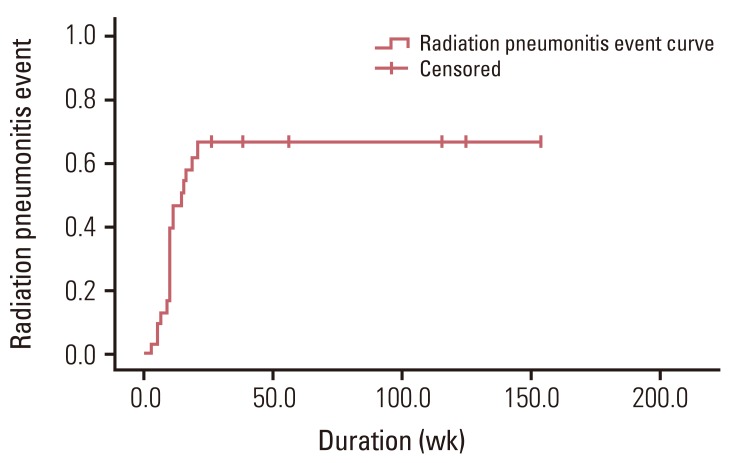
During the follow-up period, all patients experienced RP. Grade 1 RP developed in 12 patients (38.7%), grade 2 in 17 patients (54.8%), and grade 3 in two patients (6.5%). There was no grade 4 or more RP. The crude incidence rate of RP event was 61.3% (19/31). The median duration from the date of RT start to the development of RP event was 10.7 weeks (range, 3.0 to 20.9 weeks). The 2-, 4-, and 6-month actuarial RP event rates were 13.2%, 58.5%, and 67.0%, respectively (Fig. 1).
3. Predictive factors for RP event
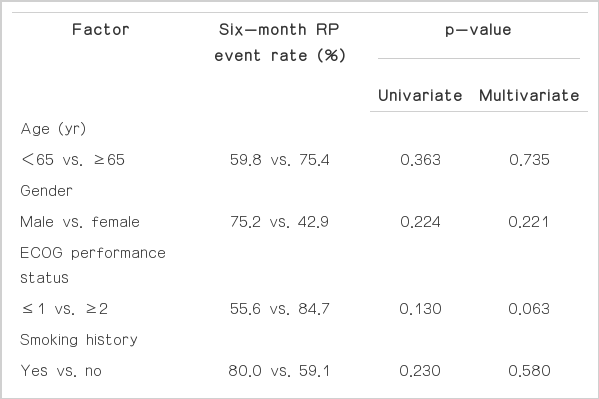
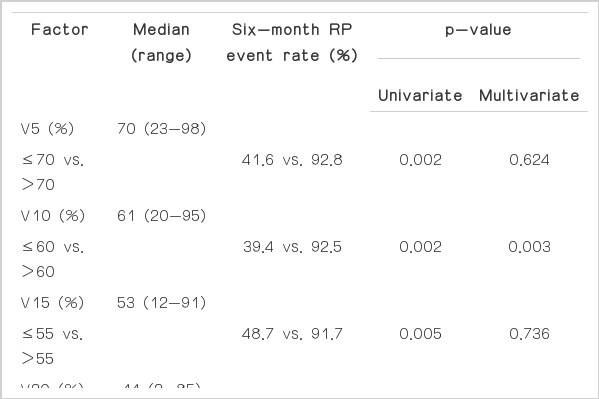
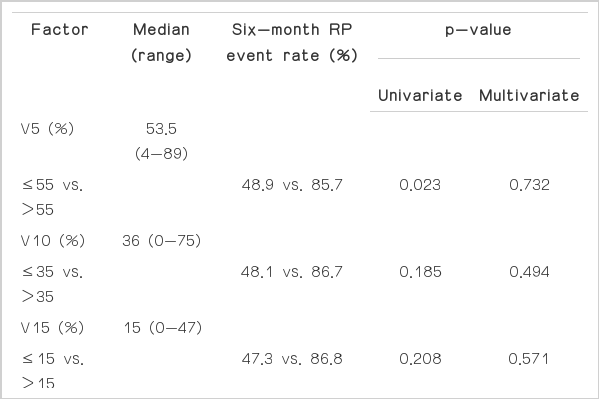
Predictive factors for RP event were analyzed. None of the clinical and treatment-related factors were associated with the incidence of RP event on univariate and multivariate analysis (Table 2). Of the dosimetric parameters, ipsilateral lung (IL) V5 (p=0.002), V10 (p=0.002), V15 (p=0.005), and CL V5 (p=0.023) were significantly associated with RP event on univariate analysis. On multivariate analysis, only IL V10 remained a significant predictive factor for RP event (hazard ratio, 4.612; 95% confidence interval, 1.604 to 13.263; p=0.003) (Tables 3 and 4).
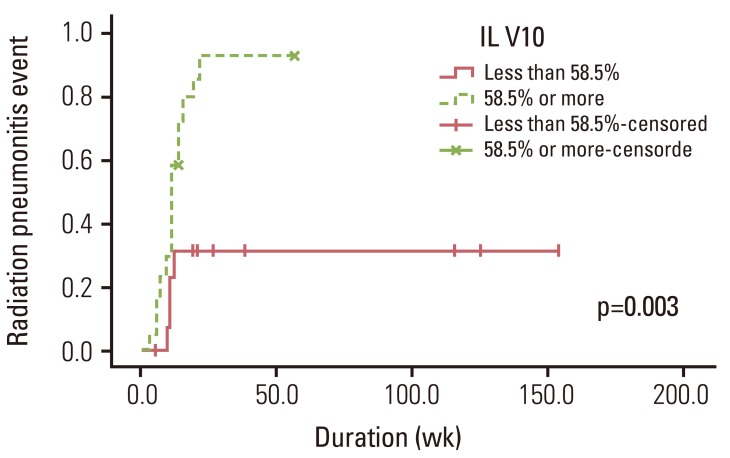
The cutoff value of IL V10, as analyzed by the ROC curves, was 58.5% (sensitivity, 78.9%; specificity, 83.3%). The 6-month actuarial incidence of RP event was 31.6% if IL V10 <58.5% and 93.1% if IL V10≥58.5% (p=0.003) (Fig. 2). The cutoff values of parameters that were significantly associated with RP event on univariate analysis were also calculated by ROC curves. The cutoff values of IL V5, IL V15, and CL V5 were 67.5% (sensitivity, 78.9%; specificity, 75.0%), 50.0% (sensitivity, 68.4%; specificity, 66.7%), and 55.5% (sensitivity, 63.2%; specificity, 83.3%), respectively.
Discussion
Because of the different scoring criteria for RP, heterogeneous patient population, heterogeneous tumor characteristics, different indications for adjuvant chemotherapy, different RT modalities and techniques, and various RT dose fractionation schedules, it is hard to compare the incidence and severity of RP among published studies. Recently, Jiang et al. [17] reported an RP incidence rate after retrospective review of 165 lung cancer cases treated by IMRT with or without chemotherapy. In their study, grade 1 RP developed in 41%, grade 2 in 24%, grade 3 in 12%, and grade 5 in 1% of cases. The 6- and 12-month actuarial grade≥3 RP rates were 11% and 14%, respectively. In our study, grade 1 RP developed in 12 patients (38.7%), grade 2 in 17 patients (54.8%), and grade 3 in two patients (6.5%). The 2-month actuarial RP event rates were 13.2%, 4-month was 58.5%, and 6-month was 67.0%. The results of our study are similar to those of Song et al. [15] who retrospectively analyzed data from 37 patients with lung cancer. In their study, as in ours, all patients were treated by HT with or without chemotherapy. The crude rate of grade 1 RP was 32%, grade 2 was 51%, and grade 3 was 8%. However, in our study, unlike that of Song et al. [15], the CTCAE 4.0 was used to score the severity of RP. The CTCAE 4.0 includes more specific descriptors for the various grades of pulmonary toxicity, making it easier to distinguish between grades 2, 3, and 4 pnuemonitis. We believe that grading the severity of RP with CTCAE 4.0 is more precise.
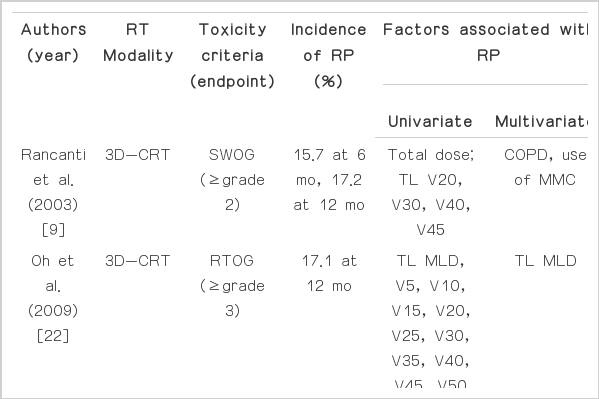
Several studies have reported predictive factors for RP, but most were retrospective, and their results were inconsistent [3,5,6,8,9,15,18-25]. Moreover, most of these studies analyzed the patient group treated with 3D-CRT or linac-based IMRT. Because almost the entire lung is exposed to a low dose of radiation when treated with HT, predictive factors for RP after HT may differ from those after 3D-CRT or linac-based IMRT. To our knowledge, there has only been one published study on the predictive factors for RP after thoracic HT. Song et al. [15] included 37 patients with lung cancer who were treated with HT in their analysis and reported that ECOG performance status, tumor location, TL dosimetric parameters (MLD, V5, V10, V13), IL dosimetric parameters (MLD, V5, V10, V13, V15, V20), and CL dosimetric parameters (MLD, V5, V10, V13) were significantly associated with grade ≥3 RP on univariate analysis. On multivariate analysis, CL V5 was the only significant independent predictive factor for development of grade ≥3 RP. In our study, IL V5, IL V10, IL V15, and CL V5 were significantly associated with grade≥2 RP on univariate analysis. On multivariate analysis, only IL V10 remained a significant predictive factor for development of grade ≥2 RP (Table 5). In our study, there were some inconsistent findings compared with Song et al.'s study [15]. Clinical factors such as performance status and tumor location were not associated with development of RP, and IL V10 were significant predictive factor for RP on multivariate analysis. However, there were important similar findings in these two studies. In contrast to other research that analyzed lung cancer groups treated with 3D-CRT or linac-based IMRT, Song et al.'s study [15] and ours reported CL V5 was a significant predictive factor for RP after thoracic HT. Because of the helical radiation delivery method in HT and consequent low-dose exposure of almost the entire CL, CL V5 is believed to be an important predictive factor for RP after thoracic HT. Therefore, to reduce the development of RP after HT in lung cancer, dose restriction for the CL might be important. Song et al. [15] reported that the cutoff value of CL V5 was 60%. In our study, we propose that CL V5 should be kept lower than 55.5% to reduce the development of RP after HT in patients with lung cancer.
There were some limitations in this study. First, this study was retrospective and therefore may have inherent bias. For example, RT dose fractionation schedules were decided according to the attending physician's discretion rather than a predetermined protocol. Thus, the dose fractionation schedule varied widely. In addition, because of incomplete patient medical records, we could not analyze some potential predictive factors for RP, such as TL dosimetric parameters. Second, the sample size was small. Third, the patient and tumor characteristics were heterogeneous. Despite these limitations, we believe that our study contributes to the identification of predictive factors for RP after HT in patients with lung cancer.
Conclusion
In our study, after HT for management of lung cancer, grade 1 RP developed in 38.7%, grade 2 in 54.8%, and grade 3 in 6.5% of all patients. There was no grade 4 or more RP. IL V5, V10, V15, and CL V5 were significantly associated with RP, and the cutoff values were 67.5%, 58.5%, 50.0%, and 55.5%, respectively.
Notes
Conflict of interest relevant to this article was not reported.