Cyclosporine A as a Primary Treatment for Panniculitis-like T Cell Lymphoma: A Case with a Long-Term Remission
Article information
Abstract
Subcutaneous panniculitis-like T cell lymphoma (SPTL) is a distinctive cutaneous lymphoma characterized by an infiltration of subcutaneous tissue by neoplastic T cells, similar to panniculitis. It is well-established that patients who are diagnosed with SPTL usually respond poorly to chemotherapy, showing fatal outcome. As a first line treatment for SPTL, anthracycline-based chemotherapy was most frequently used. For the treatment of SPTL, the efficacy of cyclosporine A has been recently reported in relapsed SPTL after anthracycline-based chemotherapy. However, it is still not clear whether cyclosporine A can be used as a first-line treatment against SPTL. Here, we report a case of SPTL, which achieved complete remission for nine years after first-line cyclosporine A therapy. This study suggests that cyclosporine A can induce a complete long-term remission as a first-line treatment.
Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is a rare type of lymphoma characterized by an infiltration of subcutaneous tissue by neoplastic T cells. It is usually presented as subcutaneous masses or flat plaques, which resemble panniculitis in appearance. It mainly involves the extremities and trunk [1]. The diagnosis of SPTL is based on cellular atypia of T cells, as well as presence of mitosis and tissue necrosis. It is well-established that patients diagnosed with SPTL usually respond poorly to chemotherapy. As a first line treatment for SPTL, anthracycline-based chemotherapy was most frequently used [1-3]. Based on recent case studies regarding SPTL, the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) suggested that SPTL is a heterogeneous disease entity with alpha/beta and gamma/delta subtypes [4]. However, there is still little evidence to suggest a definite therapeutic guideline for each subtype because the treatment outcome is similar regardless of the T cell receptor phenotype disease, although there is a significant difference in complete remission (CR) rate. A recent study has suggested that cyclosporine A has some efficacies on relapsed SPTL after combination chemotherapies [5]. We have shown that cyclosporine A induced a long-term remission in a patient with SPTL, who relapsed after anthracycline-based chemotherapy, and that the second trial of cyclosporine A was also successful in inducing a long-term remission [6]. However, it is still unclear whether cyclosporine A can be used as a first-line treatment, and whether it can induce a long-term remission. Here, we describe the first case of SPTL, which achieved a long-term CR (nine years) with cyclosporine A as a first-line therapy.
Case Report
On October 2001, a 40-year-old man visited our hospital due to intermittent fever and chills for the past four consecutive days. He had a month-long history of a lump on his right buttock. On physical examination, he had non-tender erythematous nodules on his right buttock and each leg (a total of three). Complete blood cell count (CBC) showed mild anemia and leukopenia (hemoglobin [Hb] 11.5 g/dL, white blood cell [WBC] 2.68×109/L, platelet 192×109/L). Biochemical tests revealed serum levels of aspartate aminotransferase (AST) as 261 IU/L, alanine aminotransferase (ALT) as 122 IU/L, lactate dehydrogenase (LDH) as 887 IU/L, and serum β2-microglobulin as 4,290.80 mg/L; these levels were all above their respective normal range (Fig. 1A). The skin biopsy revealed a diffuse infiltration of the subcutaneous tissue by atypical lymphoid cells (Fig. 2A). The overlying dermis and epidermis were uninvolved. The atypical lymphocytes had irregular and hyperchromic nuclei. There was rimming of atypical lymphoid cells, which surrounded the individual fat cells, and the occasional karyorrhectic nuclear debris was present between the fat cells (Fig. 2A, inlet). The lymphoid cells were positive for CD3 (Fig. 2B) and CD45RO, while negative for CD20 and CD56 (Fig. 2C and D, respectively). No T-cell receptor gamma gene rearrangement was observed. Chest X-ray, computed tomography scan of abdomen, and bone marrow biopsy revealed no involvement of other organs. There was no definite evidence of hemophagocytosis on the bone marrow examination, while leucopenia was noted on CBC. On the 13th day of his admission, cyclosporine A (400 mg/day) was given as a primary treatment, and after seven weeks, we tapered cyclosporine A over five weeks by reducing 1 mg/kg a week. Fever subsided three days after cyclosporine A therapy (Fig. 1B). Two weeks after the initial treatment, skin lesions disappeared. On 4th week of treatment, CBC (WBC 4.29×109/L, Hb 12.5 g/dL, platelet 239×109/L) and biochemical tests (alkaline phosphatase 92 IU/L, AST 19 IU/L, ALT 15 IU/L, LDH 151 IU/L) were within their respective normal ranges (Fig. 1A). Hence, for the first time, CR was documented at one-month of treatment. No recurrence has been observed until now (remission duration, 9 years).
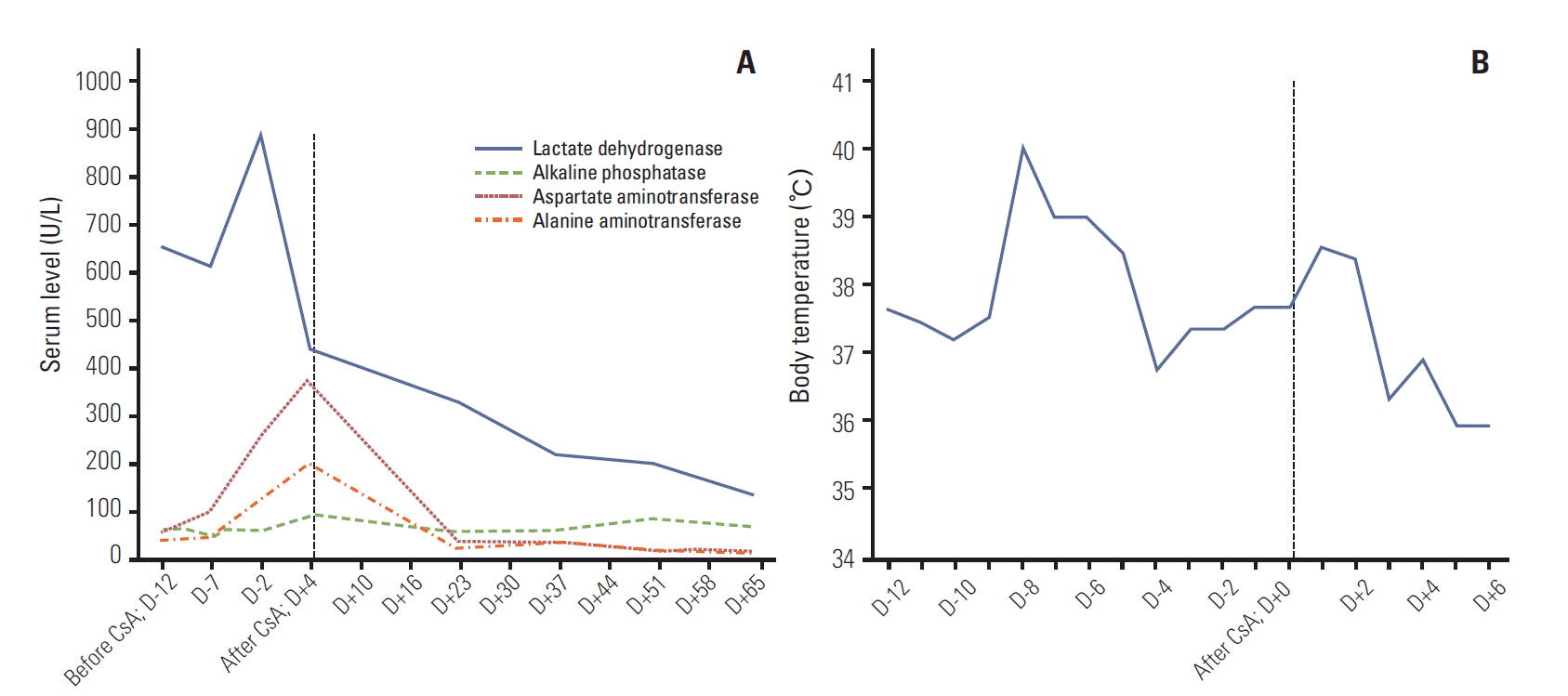
Changes in laboratory findings and body temperature during the cyclosporine A (CsA) treatment. (A) After 5 weeks of treatment initiation with CsA, biochemical test (lactate dehydrogenase, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase) was normalized. (B) After 14 days of the CsA therapy, the body temperature returned to normal.
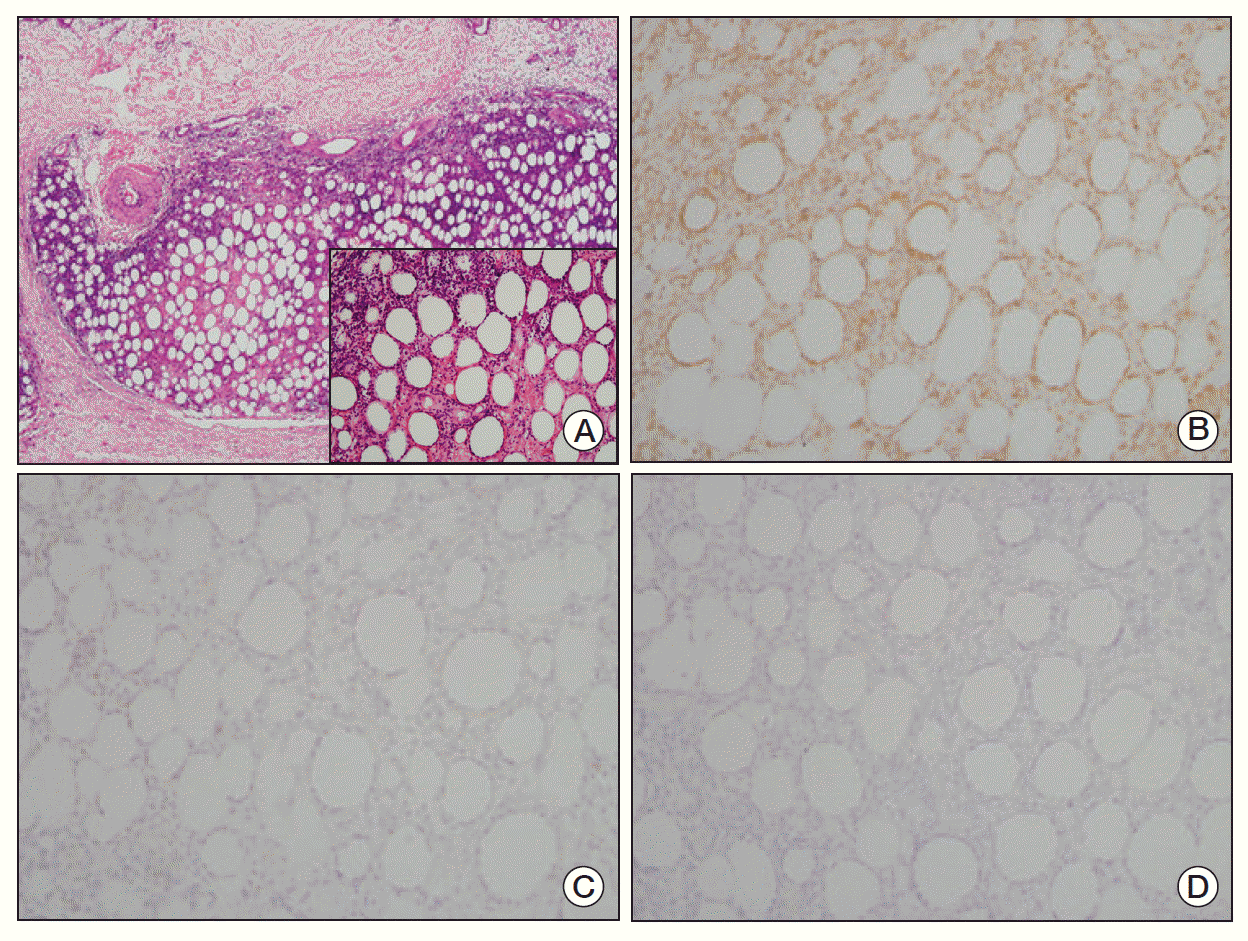
(A) The skin tissue showed diffuse atypical lymphocytic infiltration in the subcutaneous tissue similar to lobular panniculitis. Infiltration to the subcutaneous tissue without involvement of the overlying dermis or epidermis was confirmed (H&E staining, ×40). (A, inlet) The infiltrated lymphocytes had irregular, hyperchromic nuclei and indistinct nucleoli. The atypical lymphocytes were rimmed individual fat cells and some of them showed nuclear karyorrhexis (H&E staining, ×200). (B) Immunohistochemical stain, the atypical lymphocytes were positive for CD3 (×200). (C) CD20 (×200). (D) CD56 (×200). The atypical lymphocytes were negative for both CD20 and CD56.
Discussion
This case shows that cyclosporine A has induced a long-term remission as a first-line treatment without showing any side effects. Up until now, there has not been standard treatment established for SPTL due to its low incidence and lack of clinical trials. Anthracycline-based chemotherapy has been commonly used; however, the response rate is unsatisfactory [3]. Previous studies [5,7-14] demonstrated that 11 out of 14 SPTL cases were successfully treated with cyclosporine A, and 11 were induced to CR. Time to first response to cyclosporine A was within 2 weeks in most cases, regardless of prior treatments or the co-occurrence of hemophagocytic syndrome. Among these patients with SPTL, 8 out of 14 patients had a remission duration of over 6 months, and the longest was 20 months (Fig. 3). A study demonstrated that even relapsed SPTL after autologous hematopoietic stem cell transplantation can be cured by the same regimen of our case, cyclosporine A 400 mg/day [8]. For the treatment of lymphoma, treatments inducing CR with a short-term remission duration would not be considered as an effective treatment. In that respect, this case has another merit; this study clearly shows that with a long-term follow-up, cyclosporine A alone can be considered as a primary treatment for SPTL even though cytopenia and liver function test abnormality were observed that may be associated with organ involvement. In prior studies, two patients with SPTL achieved complete response, and one patient achieved partial response after the initial cyclosporine A treatment. Above all, the most important point of this study is that this was the first case that showed cyclosporine A inducing a long-term remission of over 5 years as the initial treatment for SPTL. Actually, the patient in this report achieved a CR for more than nine years. Because cyclosporine A is less toxic when compared to anthracyclinebased chemotherapy or a long-term steroid therapy and has a dramatic efficacy in some cases, cyclosporine A can be a good option as the primary treatment for SPTL. In conclusion, this study suggests that cyclosporine A can induce a complete long-term remission as a first-line treatment. To confirm this finding and make a good guideline for the treatment of SPTL, a large-scale randomized study is warranted.
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This study was in part supported by a grant of Korean Cancer Research Institute.
