Efficacy and Safety of Oxaliplatin, 5-Fluorouracil, and Folinic Acid Combination Chemotherapy as First-Line Treatment in Metastatic or Recurrent Gastric Cancer
Article information
Abstract
Purpose
We retrospectively determined the efficacy and safety of the combination of oxaliplatin, 5-fluorouracil (5-FU), and folinic acid (FA) as first-line chemotherapy for patients with metastatic or recurrent gastric cancer.
Materials and Methods
Between January 2006 and August 2009, 39 patients with histologically-confirmed, metastatic or recurrent gastric cancer underwent chemotherapy, and the results were retrospectively investigated. The chemotherapy regimen consisted of oxaliplatin (100 mg/m2) and FA (200 mg/m2; 2-hour infusion), then 5-FU (2,400 mg/m2; 46-hour continuous infusion) every 2 weeks.
Results
Thirty-nine patients received a total of 210 treatment cycles. The median number of cycles was 6 (range, 1 to 16). Of the 32 evaluable patients, zero patients achieved a complete response and 11 patients achieved a partial response (response rate, 28.2%). The median time-to-progression and overall survival were 4.3 months (95% confidence interval [CI], 2.0 to 6.5 months) and 9.8 months (95% CI, 3.5 to 16.0 months), respectively. The main hematologic toxicity was anemia, which was observed in 119 cycles (56.7%). Grade 3/4 neutropenia was observed in 32 cycles (15.2%). The main non-hematologic toxicity was constipation, which was observed in 91 cycles (46.2%). Peripheral neuropathy occurred in 71 cycles (33.8%); all cases were grade 1 or 2. No treatment-related deaths were reported.
Conclusion
This study showed that combination chemotherapy with oxaliplatin, 5-FU, and FA is an active and well-tolerated regimen as first-line treatment in patients with metastatic or recurrent gastric cancer.
Introduction
Despite the declining incidence of gastric cancer in Western countries, gastric cancer is the most frequently diagnosed cancer worldwide, and is one of the main causes of cancer deaths [1]. In patients with advanced gastric cancer with distant metastasis or recurrence, systemic chemotherapy has been recommended as standard treatment, and the prolongation of survival or increase in the quality of life is well-established [2]. However, patients with advanced gastric cancer with distant metastasis or recurrence remain incurable, and these patients have a median survival of only 7-13 months, despite receiving chemotherapy [3]. Also, first-line standard chemotherapy regimens have not been defined for patients with advanced gastric cancer. Cisplatin-based regimens have demonstrated superior efficacy compared to platinum-free regiments, although cisplatin-based regimens have significant toxicity [4,5].
Oxaliplatin is a third generation platinum compound with a lack of cross-drug resistance with cisplatin, a synergistic effect with 5-fluorouracil (5-FU), and a satisfactory safety profile compared to cisplatin [6-9]. Moreover, many adducts of oxaliplatin appear to be more effective in inhibiting DNA synthesis than adducts of cisplatin [10-12]. Two phase III trials have directly compared oxaliplatin-based versus cisplatin-based regimens [13,14], both of which have concluded that oxaliplatin is at least as effective as cisplatin, and has a lower toxicity profile, except neurotoxicity, in combination regimens for patients with advanced gastric cancer. In several phase II studies involving the treatment of advanced gastric cancer, various combinations of oxaliplatin, 5-FU, and folinic acid (FA), as first- or second-line treatments, have shown considerable activity [15-19]. However, the optimal dose and schedule of combination oxaliplatin, 5-FU, and FA were not established. The aim of this retrospective study was to report the efficacy and toxicity of a biweekly protocol of oxaliplatin (100 mg/m2) combined with 5-FU (2,400 mg/m2; 46-hour continuous infusion), and FA (200 mg/m2) as first-line treatment on response, survival, and toxicity rates of metastatic and recurrent gastric cancer in Korean patients.
Materials and Methods
1. Eligibility criteria
The eligibility criteria were as follows: 1) histologically-confirmed metastatic or recurrent adenocarcinoma of the stomach and ≥18 years of age; 2) no previous chemotherapy, except post-operative adjuvant chemotherapy or chemoradiation that was received >6 months previously; 3) an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; 4) no serious or uncontrolled concurrent medical illness; 5) at least one measurable lesion; and 6) adequate baseline hematologic function (absolute neutrophil count [ANC]≥1.5×109/L, platelet count≥100×109/L), adequate hepatic function (serum bilirubin≤1.25×upper normal limit [UNL], serum aspartate aminotransferase, alanine aminotransferase≤2.5×UNL, serum alkaline phosphatase≤5.0×UNL), and adequate renal function (serum creatinine≤1.5 mg/dL). The exclusion criteria were as follows: 1) preexisting peripheral neuropathy; 2) concurrent or prior malignancy, except curative resection of cervical carcinoma in situ or squamous cell carcinoma of the skin; 3) active infection; and 4) concurrent treatments that interfered with the evaluation of the study.
2. Treatment protocol and dose modifications
1) Chemotherapy protocol
Oxaliplatin (100 mg/m2) and FA (200 mg/m2) were given as a 2-hour intravenous infusion followed by 5-FU (2,400 mg/m2) as a 46-hour continuous infusion every 2 weeks. Treatment was continued until disease progression, unacceptable toxicity, patient refusal, or at the discretion of the physician. Anti-emetic prophylaxis was given according to a local protocol. Granulocyte colony-stimulating factor was not routinely used in the study.
2) Treatment delays and dose modifications
Each cycle was started if the ANC and platelet count on the day of treatment were >1.5×109/L and >100×109/L, respectively. In the case of toxicity ≥ grade 3, except alopecia, chemotherapy was delayed 1 week, then restarted after full recovery. A reduction of 25% in all drug doses was applied to grade 3/4 thrombocytopenia or febrile neutropenia, grade 4 neutropenia, and grade 3/4 non-hematologic toxicity in the previous cycle of chemotherapy.
3. Response and toxicity assessment
The response to treatment was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (ver. 1.0) [20]. Computed tomography (CT) scans of the measurable lesions were performed within 2 weeks prior to treatment. After every 4th cycle of chemotherapy, CT scans were performed to determine the response of the disease, or sooner if there was evidence of any clinical deterioration. Complete blood cell counts with differentials and serum biochemistries were repeated prior to the start of each chemotherapy cycle, and the toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC, ver.3.0).
4. Statistical analysis
Overall survival (OS) was calculated from the first day of chemotherapy to the date of death. Time-to-progression (TTP) was calculated from the first day of chemotherapy to the date of disease progression. The Kaplan-Meier method was used to analyze the TTP and OS, and the 95% confidence interval (CI) for the median time-to-event was computed. The Cox proportional hazard method was used for assessing independent prognostic factors. Statistical significance was calculated at the 95% CI (p<0.05). All the analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
Between January 2006 and August 2009, 39 patients with a median age of 62 years (range, 38 to 78 years) were retrospectively studied in Seoul, Bucheon, and Cheonan Soonchunhyang University Hospitals. There were 22 men and 17 women, and 2 patients (5.1%) had an ECOG performance status of 2. Thirty-one patients (79.5%) had primary metastatic disease and 8 patients (20.5%) had recurrent disease. Lymph nodes were the most common metastatic sites. The metastasis involved two organs in 11 patients (28.2%) and >3 organs in 11 patients (28.2%). Eight patients who had previously undergone curative surgery received adjuvant 5-FU-containing chemotherapy. The majority of the patients (71.8%) had 1 or 2 involved regions, and the common target lesion was a lymph node or the liver. The patient baseline characteristics are listed in Table 1.
2. Tumor responses and survival
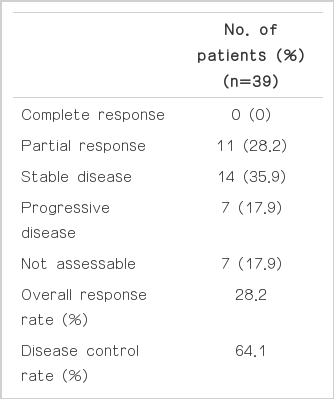
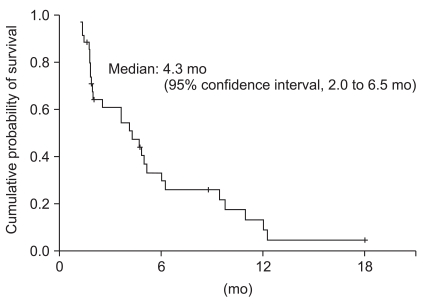
Thirty-two of the 39 patients were evaluable for response according to the RECIST criteria (ver. 1.0). The response was not assessable in 7 patients who were lost to follow-up (n=3), voluntary refusal for further treatment after 1 or 2 cycles (n=2), and death before the 4 cycles of chemotherapy (n=2). Eleven patients (28.2%) achieved a partial response, 14 patients (35.9%) had stable disease, and 7 patients (17.9%) had progressed during the course of treatment (Table 2). None of the patients achieved a complete response. On intention-to-treat analysis, the overall response rate (ORR) and disease control rates were 28.2% (95% CI, 11.7 to 44.7%) and 64.1% (95% CI, 49.9 to 78.3%), respectively. The median duration of follow-up was 20.1 months (range, 2.1 to 63.0 months). The TTP and OS were 4.3 months (95% CI, 2.0 to 6.5 months) (Fig. 1) and 9.8 months (95% CI, 3.5 to 16.0 months) (Fig. 2), respectively.
3. Factors affecting survival and response
An analysis of prognostic factors affecting survival was attempted. Then, age (0-64 years vs.>65 years), gender, a history of surgery or chemotherapy, ECOG performance status (0-1 vs. 2), disease status before chemotherapy (newly diagnosed vs. recurrent), and the number of metastases (0-2 vs.>3) were compared between the two groups. Based on univariate analysis, the ECOG performance status (p=0.004) and the number of metastases (p=0.033) were significantly different between the two groups. Only the ECOG performance status was a significant factor affecting survival based on multivariate analysis (hazard ratio, 0.134; p=0.043). However, there were no significant factors affecting response.
4. Toxicity and dose intensity
Two hundred ten chemotherapy cycles were administered to the 39 patients. The median number of cycles was 6 (range, 1 to 16). Table 3 shows the summaries of the incidence of main toxicities. The major grade 3 or 4 hematologic toxicities per cycle were neutropenia (15.2%) and thrombocytopenia (7.6%). Two patients (5.1%) had febrile neutropenia. The major non-hematologic toxicities per cycle were constipation (46.2%) and nausea (41.9%). Peripheral neuropathy per cycle occurred in 33.8% of the patients and all cases were grade 1-2; there were no treatment interruptions or discontinuations due to neuropathy. One patient (2.6%) had acute renal failure after 1 treatment cycle, which required transient hemodialysis. The relative dose intensities of oxaliplatin and 5-FU were 87.4% and 86.2% of the planned doses, respectively. No treatment-related deaths occurred.
Discussion
Despite a large number of randomized trials, the best agent or optimal regimen for the first-line treatment of advanced gastric cancer has not been determined. A variety of different oxaliplatin combination regimens have been studied (oxaliplatin plus 5-FU, epirubicin, or capecitabine) in phase II trials. Also, several studies involving various combinations of oxaliplatin/5-FU/FA for treating advanced gastric cancer have been published; these studies have shown consistent results regarding the activity of oxaliplatin combinations [15-19]. The range of TTP, median OS, and ORR of these trials were 4.3-6.2 and 7.3-9.6 months, and 26-45%, respectively. Although data from randomized controlled phase III trials have not been published, in practice various combinations of oxaliplatin/5-FU/FA are used for the treatment of advanced gastric cancer in Korea. However, one prospective study involving oxaliplatin/5-FU/FA as first-line chemotherapy in Korean patients with advanced gastric cancer has been conducted [21]. In that study, all 37 patients were elderly (>65 years of age). The objective of our study was to assess the efficacy and toxicity of a biweekly combination of oxaliplatin (100 mg/m2 in a 2-hour infusion), FA (200 mg/m2 in a 2-hour infusion), and 5-FU (2,400 mg/m2 in a 46-hour continuous infusion) as first-line treatment for treating younger patients with advanced gastric cancer in Korea. Although our study was retrospective, the results showed that the chemotherapy regimen was an effective and safe combination for advanced gastric cancer as first-line treatment in Korea, as well as Western countries. The OS of 9.8 months was within the range of previous trials (7-12 months) using platinum-containing and taxane- or irinotecan-based regimens. Although survival is not a reliable point for evaluation of efficacy in a retrospective study, the median survival of 9.8 months in the current study compared favorably with previous studies [3]. The treatment compliance for this regimen was good, with a dose adherence to both oxaliplatin and 5-FU of >85%. Owing to the patient selection bias, a comparison of the toxicity profiles in this study with other phase II studies that used various oxaliplatin and 5-FU administration schedules is not relevant. Compared with previous biweekly regimens [15-17], the biweekly protocol in the current study used higher levels of oxaliplatin (100 mg/m2 vs. 85-100 mg/m2), and lower levels of FA (200 mg/m2 vs. 400-500 mg/m2) and 5-FU (2.4 g/m2 vs. 2.4-3.0 g/m2). In the current study, major grade 3/4 hematologic toxicity, including neutropenia (30.8%) and thrombocytopenia (20.0%), was encountered. Although we infused 5-FU continuously without bolus to reduce hematologic toxicity, the rates of grade 3/4 neutropenia and thrombocytopenia were relatively high compared with other studies [16,18,19]. It is possible that the rate of hematologic toxicity reflected the relatively high dose of oxaliplatin; however, to make such an assumption, more evidence should be provided, such as 85 mg/m2 vs. 100 mg/m2 of oxaliplatin in Caucasian and Asian populations. Nevertheless, the toxicities were manageable. Neurotoxicity is the most common side effect in an oxaliplatin-containing protocol, but there was no grade 3/4 neurotoxicity encountered during the current study, which may have been due to the relatively low cumulative dose of oxaliplatin (median, 521 mg/m2) used in this setting; indeed, neuropathy is particularly prevalent for cumulative doses >540 mg/m2 [22]. One female patient had acute renal failure after one treatment cycle requiring transient hemodialysis; she had normal renal function before treatment and did not receive medications with renal toxicity. Further, she declined continuation of treatment for voluntary withdrawal after one cycle. Therefore, the investigator considered renal toxicity a possible untoward effect related to oxaliplatin [16]. In the current study, diarrhea occurred in 6.7% of the cycles, but 46.2% of the cycles had associated constipation (grade 1 or 2). Compared with the other oxaliplatin/5-FU/LV regimens studied in gastric cancer, the rate of constipation was high [16,19]. It would be worthwhile to determine whether or not oxaliplatin would slow the intestinal transit time in Asians.
Conclusion
Although our study was retrospective, biweekly combination chemotherapy with oxaliplatin, 5-FU, and FA was well-tolerated and active as first-line treatment in patients with metastatic or recurrent gastric cancer.
Notes
Conflict of interest relevant to this article was not reported.