INTRODUCTION
The optimal treatment of patients with locally-advanced unresectable non-small cell lung cancer (NSCLC) involves a combination of chemotherapy and thoracic radiotherapy (TRT). TRT alone rarely achieves long-term survival in excess of 5-10% and is frequently associated with local and/or distant failures (1). To achieve further improvements in outcomes, the addition of chemotherapy, prior to or concurrent with TRT, has been evaluated (2~4). However, the most efficacious combination of chemotherapeutic agents, as well as their dose and schedule, remain to be established.
Paclitaxel (Taxol®, Bristol-Myers Squibb) has significant activity in NSCLC when used alone or in combination (5). Paclitaxel also enhances the effects of radiation through its effects on cell cycle distribution as well as on reoxygenation of radioresistant hypoxic cells (6,7). In view of these radiosensitizing effects and its ability to synergize with cisplatin (8), paclitaxel has been widely incorporated into platinum-containing chemoradiation regimens (9).
To enhance the potential of paclitaxel for radiosensitization, more frequent administration of paclitaxel has been proposed. A 60% overall response rate was reportedly achieved with weekly paclitaxel plus cisplatin and concurrent TRT (10). Herein, the results of a phase II study of weekly paclitaxel, cisplatin and concurrent TRT for locally-advanced unresectable NSCLC are reported.
MATERIALS AND METHODS
1) Patient eligibility
Eligible patients were required to have a histologically or cytologically confirmed, locally-advanced unresectable stage III NSCLC and the primary tumor and/or metastases had to be measurable by imaging studies. Other eligibility criteria were: aged under 75 years; an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or lower; adequate bone marrow (hemoglobin >10 g/L, neutrophil count >1,500/mm3 and platelet count >100,000/mm3), pulmonary (FEV1 > 1 L), hepatic (a serum total bilirubin level <1.5 mg/dl, AST/ALT < ×2.5 ULN) and renal functions (a serum creatinine level <1.5 mg/dl). No prior chemotherapy or radiation therapy was allowed. Patients with any evidence of malignant pleural effusion, cervical lymphadenopathy, or distant metastasis were excluded. Patients were also excluded from the study if they had a severe co-morbid illness or known history of anaphylaxis of any origin. The nature and purpose of the study was fully discussed with each patient, and each provided written informed consent before registration.
2) Treatment
Before registration, a complete medical history, physical examination and imaging studies, including chest radiography, computed tomography of the chest and upper abdomen and skeletal scintigraphy, were performed to rule out distant metastasis. Magnetic resonance imaging of the brain was required if symptoms suggested central nervous system involvement.
The chemotherapy consisted of paclitaxel 40 mg/m2, in a 3-hours infusion, followed by cisplatin 20 mg/m2. Both were given on days 1, 8, 15, 22, 29, 36 and 43 of the planned TRT course. The dosage in the subsequent cycles was adjusted according to the toxic effects that developed during the preceding cycle. TRT was delivered simultaneously on the first day of chemotherapy. The planned total dose was 63 Gy of radiation, at 1.8 Gy/day, in 35 fractions over 7 weeks. Patients who had not progressed by completion of the TRT were to receive 2 additional cycles of consolidation chemotherapy with paclitaxel 175 mg/m2 and cisplatin 75 mg/m2 on day 1 of a 3-weeks cycle. Treatment on an outpatient basis was allowed, and all patients received standard supportive regimen consisting of adequate hydration, dexamethasone, antihistamines and antiemetics.
3) Evaluation
Follow-up history, physical examinations and toxicity assessment were performed before each cycle of chemotherapy. For the radiation period, chest radiography, complete blood counts and serum chemistries were performed weekly. A computed tomography of the chest and upper abdomen was repeated 3 weeks after completion of the TRT and 3 weeks after the additional 2 cycles of consolidation chemotherapy.
The tumor response and toxicity were recorded in accordance with the World Health Organization (WHO) criteria. Patients were followed every month after completion of the protocol treatment until tumor progression, onset of severe, unusual toxicity or death occurred.
4) Statistics
The primary end point of the trial was the efficacy of the therapy, as measured by the objective response rate and time to progression, with toxicity as a secondary end point. To minimize the expected accrual into unacceptable treatment, either in terms of clinical response or toxicity, sample size and decision criteria were calculated with a 2-stage design method (11). After testing the treatment on 17 patients in the first stage, the study would be terminated if 10 or fewer patients responded or 7 or more patients experienced unacceptable toxicity (i.e., treatment-related death or early discontinuation of treatment). If the study went to the second stage, a total of 35 patients would be studied to detect a 25% improvement in the objective response rate and an unacceptable toxicity probability of 60% or less (α=0.1; β=0.2).
The time to disease progression was calculated from the start of treatment to that of clinical progression. Overall survival was defined as the interval between the start of treatment to death or the last follow-up visit. The median response duration, overall survival and time to progression were calculated with 95% confidence intervals (CI). Statistical calculations were performed using SPSS software, version 11.5 (SPSS, Inc, Chicago, IL). Comparisons were performed using chi-square test and survival curves were generated using the Kaplan-Meier method. Results were considered significant at a P<0.05.
RESULTS
1) Patient characteristics
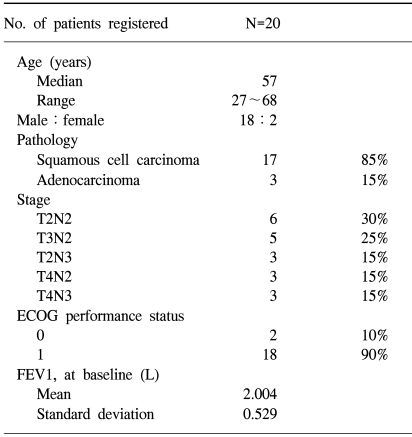
Among a total of 20 patients registered between February 2000 and December 2002, 4 were not evaluable for response due to early discontinuation of treatment. The characteristics of the patients are listed in Table 1. The median age was 57 years (range, 27 to 68) and most patients (90%) had an ECOG performance status of 1. Eleven patients had unresectable stage IIIa tumors and 9 had stage IIIb (3 by T4 and 6 by N3 designation) disease. Three patients had adenocarcinomas and 17 had squamous cell histology.
2) Treatment delivery
Patients received a median of 7.6 weeks of chemoradiation (range, 3.3 to 9.4 weeks), with a total of 102 courses of chemotherapy administered concurrently with the TRT (median, 6 courses; range, 1 to 6). Thirteen (65%) patients completed the concurrent chemoradiation as planned; two patients stopped treatment due to toxic effects, 2 declined further treatment and 3 had progression of disease while receiving chemoradiation.
3) Toxicity
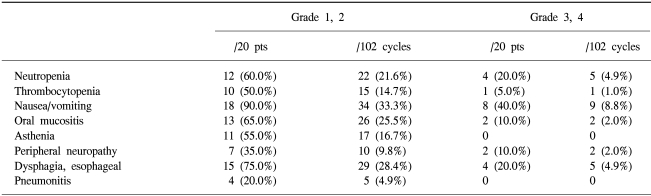
Chemotherapy was delayed in 16 out of the 102 cycles (15.7%). As the dose intensities of paclitaxel 40 mg/m2/week and cisplatin 20 mg/m2/week were planned, the relative dose intensities of each drug were 76.4% (95% CI, 57.8 to 95.0%) and 83.1% (95% CI, 66.6 to 99.5%), respectively. The hematologic and non-hematologic toxic effects are summarized in Table 2. The most frequently encountered severe toxicities were neutropenia and gastrointestinal toxicity, which were managed with rest, dose reduction, or treatment discontinuation. Febrile neutropenia occurred in 4 patients (20.0%), and a further 4 had pulmonary toxicity as determined by their symptoms and radiographic findings. Pneumonitis improved rapidly with corticosteroids and supportive care. Fifteen (75%) patients experienced at least one episode of grade 3 or 4 toxicity. In one patient, a period of dialysis was required for oliguric renal failure. No patient died during treatment.
4) Efficacy
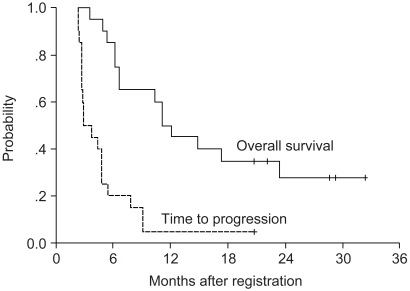
No patient achieved a complete clinical response. Of the 16 patients who were evaluable for responses, 7 partial responses were observed (43.8%; 95% CI, 19.5 to 68.1%). Taking into account the 5 patients who had stable diseases, 12 patients received at least one cycle of consolidation chemotherapy. With a median follow-up duration of 32.4 months (95% CI, 25.5 to 39.4 months), the median time to progression and overall survival were 2.8 (95% CI, 1.5 to 4.2 months) and 11.4 months (95% CI, 8.6 to 13.7 months), respectively (Fig. 1). The 1-year and 2-year actuarial survival rates were 45 and 35%, respectively.
Unsatisfactory response rates and the high incidence of grade 3 or 4 toxicities, including 7 early discontinuations of treatment, exceeded the study stopping rules, resulting in the early closure of the study.
DISCUSSION
Surgical resection is the treatment modality of choice, with the best chance to cure, in patients with NSCLC. However, patients with stage III disease have a surgical cure rate below 10% (12). The outcomes after definitive TRT alone, given with curative intent, is also poor, with a median survival of 9~11 months and 5-year survival of 3~10% (1,13). It is for this group of patients, with a locally-advanced unresectable disease, that multimodality treatment strategies have been explored, with the hope that an improvement in local and/or distant control might result in improved survival (4). Several different approaches have been developed for stage III NSCLC, including induction chemotherapy followed by TRT (14,15), concurrent chemoradiation therapy, and induction chemotherapy or chemoradiation followed by surgical resection (16,17). Combined chemotherapy and TRT appear to improve the outcome in patients with locally-advanced NSCLC, achieving a median survival of 13~14 months. A meta-analysis has demonstrated a significant survival benefit for patients receiving cisplatin-based chemotherapy combined with TRT, compared to those receiving TRT alone (18).
Recent developments of chemotherapy regimens, employing newer agents, such as taxanes, appeared more promising; however, it is apparent that the more complex a multimodality treatment, the more toxic and difficult for patients to tolerate. The goal of the current study was to evaluate efficacy and safety of weekly paclitaxel and cisplatin given concurrently with TRT. In a series of other phase II studies, which evaluated concurrent chemoradiation with paclitaxel and cisplatin, chemotherapy was administered every 3 weeks (19,20) or weekly (21,22), which resulted in objective response rates of 78 to 84%, with a median survival of 12~21 months with acceptable toxicity. Unfortunately, these experiences could not be translated into success in the current study.
Moreover, the 43.8% response rate and significant toxic effects experienced calls into question the use of a weekly bolus administration of paclitaxel and cisplatin concurrently with TRT. Even if the efficacy outcomes achieved in this study (response rate 43.8% and median survival 11.4 months) were thought favorable in patients with locally-advanced unresectable NSCLC, the pre-designed criteria for continuing the trial were not reached. No factors could be identified that may help explain the unexpected poor outcome, compared to results of other reports, of the patients with locally-advanced unresectable NSCLC treated in this study. One possible explanation is that the dose intensities chosen for this study were lower than recommended in the phase I trial (10). Due to the low (65%) compliance with the planned treatment and the high proportion of symptomatic patients with an ECOG performance status of 1, the dose or schedule was a likely factor, as protracted continuous infusion paclitaxel is more promising in terms of its feasibility and dose intensity (23). Despite the high level of clinical monitoring and supportive care given to the symptomatic registered patients, an exaggerated spectrum of toxicity was unable to be avoided.
The early closure of the current study prevented the enrollment of a sufficient number of patients to determine the activity of this concurrent chemoradiation therapy in patients with locally-advanced unresectable NSCLC. The unfavorable outcomes observed in this study raises the possibility that continuous infusion paclitaxel may be a safer option when incorporated into multimodality strategies. Therefore, a new prospective study of paclitaxel and cisplatin with concurrent TRT has been initiated, with the protocol for paclitaxel amended to 7 weeks protracted continuous infusion.
CONCLUSIONS
In this phase II study, the efficacy and safety of weekly paclitaxel/cisplatin plus TRT was evaluated in patients with locally-advanced unresectable NSCLC. An unsatisfactory response rate and the high incidence of grade 3/4 hematologic and non-hematologic toxicities, including 7 early discontinuations of treatment, exceeding the study stopping rules, prompted the early closure of the study. In view of the activity observed, the protocol was amended to protracted continuous infusion paclitaxel, cisplatin and concurrent TRT.