INTRODUCTION
CD24 has recently attracted considerable interest, as it belongs to the small group of mucin-type glycoprotein ligands for P-selectin. P-selectin is rapidly induced on the surface of platelets or endothelial cells following activation with a variety of agents (1). The human CD24 antigen is a small heavily glycosylated, glycosylphosphatidylinositol (GPI) linked cell surface protein. It consists of a small protein core comprising 27 amino acids, which is extensively glycosylated and is bound to the membrane via a phosphatidyl-inositol anchor (2). The human CD24 antigen is physiologically expressed in developing or regenerating tissue, as well as in granulocytes, keratinocytes and renal tubular epithelium. It is normally expressed on the surface of the B cell precursor, but its expression is lost with the onset of plasma cell differentiation. In neoplasia, its expression has been described initially in hematological malignancies, but also in a large variety of solid tumors, including renal cell carcinoma, small cell lung carcinoma, nasopharyngeal carcinoma, hepatocellular carcinoma, bladder carcinoma, glioma, breast cancer and ovarian cancer (3). Recent studies have shown that in ovarian cancer the mean survival time for patients with tumors showing cytoplasmic CD24 staining is less than half that of patients with tumors which do not show cytoplasmic CD24 staining (4). Similar observations were reported for breast (5) and prostate carcinomas (6).
In this study, we examine the consecutive cases of gastric cancer and evaluate the relationship between CD24 expression and the clinicopathologic data including patients' survival.
MATERIALS AND METHODS
1) Patients and samples
A total of 300 consecutive, surgically resected cases of gastric carcinomas were identified from the files of the Department of Pathology, Seoul National University College of Medicine. The age, gender, tumor location, lymphatic invasion, vascular invasion and pTNM stage were evaluated by reviewing the medical charts and pathological records. Glass slides were reviewed for histological classification according to the WHO and the Lauren classification systems. The mean age of the patients was 54.3 years. No patient had received preoperative chemo- or radiotherapy, and 92.7% (278/300) of the patients had undergone curative resection (R0 according to the UICC guideline). The clinical outcome of the patients was followed from the date of surgery to either the date of death or December 1, 2000, resulting in a follow-up period ranging from 1~72 months (mean: 53 months). Those cases lost to follow-up and those ending in death from any cause other than gastric cancer were regarded as censored data during the analysis of the survival rates.
2) Immunohistochemistry
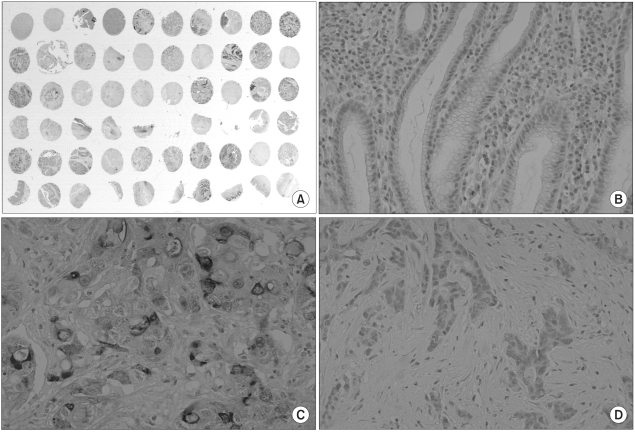
The protein expression of CD24 was assessed by an immunohistochemical staining method using a tissue array (Superbiochips laboratories, Seoul, Korea). Core tissues biopsies (2 mm in diameter) were taken from individual paraffin-embedded gastric tumors and seeded in a new recipient paraffin block (tissue array block) using a trephine apparatus. Each tissue array block consisted of 60 samples, and each block contained normal gastric mucosa from the body, antrum and cardia. Sections of 4µm thickness were cut from each tissue array block.
After the sections were deparaffinized and dehydrated, and immunohistochemical staining with antibody against CD24 (mouse monoclonal antibody; Neomarkers, Fremont, CA, USA) was performed using a streptavidin-biotin peroxidase procedure, following an antigen retrieval process with microwaves. To prevent the decay of antigenicity, immunohistochemistry was performed within a week after slide preparation. In the previous experiment, we studied the expression of several antigens using the same tissue array slides (7,8). We obtained the expression results of PTEN (anti-PTEN, AG Scientific, San Diego, CA) and CD44 (anti-CD44, Novocastra, Newcastle, UK) from the previous study, and compared the result with the CD24 expression.
3) Statistical analysis
For the clinicopathologic analysis, the chi-square test or Fisher's exact test (two sided) was performed. The cumulated survival curves were constructed by the Kaplan-Meier method and differences between the curves were tested for significance using the log-rank test. Multivariate Cox proportional hazard models were used to estimate the hazard ratios and 95% confidence intervals (CI). We included CD24 expression, sex, pathologic staging, and histologic parameters (Lauren's classification, lymphoid stroma and lymphatic invasion) in the multivariate analysis. The results were considered to be statistically significant when the p values were less than 0.05. All statistical analyses were conducted using the SPSS 11.0 statistical software program (SPSS, Chicago, IL).
RESULTS
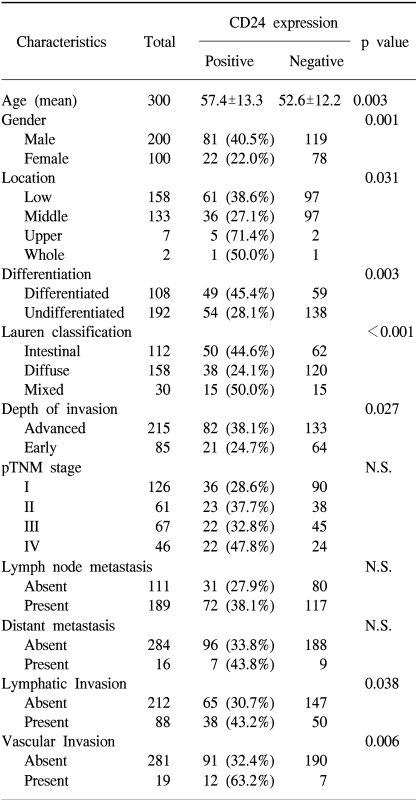
The non-neoplastic gastric mucosa did not show CD24 expression. In the case of the gastric carcinomas, membranous and cytoplasmic expression of CD24 was found (Fig. 1). CD24 immunoreactivity was detected in 103 of the 300 gastric carcinomas (34.3%). The clinicopathological data of our study are presented in Table 1. The positive CD24 expression was more prevalent in the cancers of the upper portion (71.4%, p=0.031), in differentiated cancer (45.4%, p=0.003), in the intestinal subtype according to the Lauren classification (44.6%, p<0.001) and in advanced cancer (38.1%, p=0.027). The correlations with lymph node metastasis and distant metastasis were statistically insignificant, however, significant association was noted with lymphatic invasion (43.2%, p=0.038) and with vascular invasion (63.2%, p=0.006)
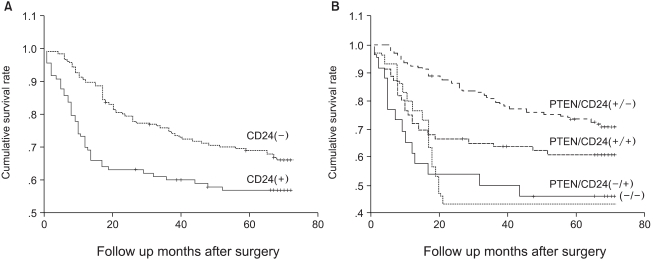
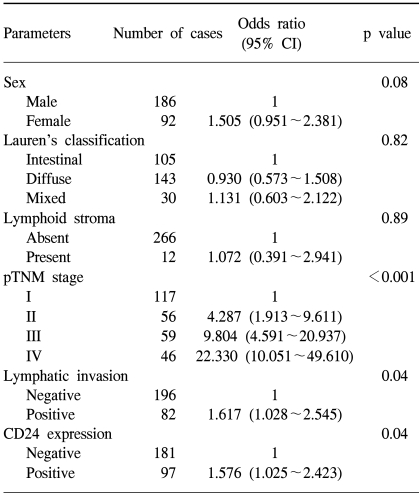
We compared the survival of patients whose tumors expressed CD24 with that of patients whose tumors did not express CD24 (Fig. 2A). The cumulative survival rate was significantly better in patients with CD24-negative tumors than in those with CD24-positive tumors (p=0.0207). By multivariate analysis, pathologic staging is the strongest prognostic parameter, and lymphatic invasion is also a prognostic factor. CD24 overexpression was found to be significantly associated with patient survival independently of the pTNM stage or lymphatic invasion (p=0.044, Table 2).
We compared the expression of CD44 and CD24, because both of them are the ligands of adhesion molecules. The expression of CD24 and CD44 is positively correlated; CD24 expression was found to be higher in CD44 (+) cases (47.1%) than in CD44 (-) cases (32.5%, p=0.048). When we compared the expression of CD24 and PTEN, they are negatively correlated; CD24 was expressed in 31.7% of PTEN (+) cases, but in 46.7% of PTEN (-) cases (p=0.031). We investigated the survival difference of each group with combinational expression of CD24 and PTEN proteins. PTEN(+)/CD24(-) cases showed the best survival (mean survival, 61 months) among the four groups (p=0.0003, Fig. 2B).
DISCUSSION
CD24 is a cell-surface glycoprotein involved in cell-cell and cell-matrix interactions. There is increasing evidence that CD24 contributes to both cellular signaling and cell adhesion(1). CD24 is expressed during progression and metastasis in ovarian (4), breast (5) and prostate cancers (6).
The results of the current study showed that CD24 immunoreactivity was detected in 38.1% of advanced carcinomas compared to 24.7% of early carcinomas (p=0.027); and in 45.4% of cases with differentiated tumors compared to 28.1% of cases with undifferentiated tumors (p=0.003). We also noted that CD24 expression in cases with lymphatic invasion was 43.2% (p=0.038). Furthermore, 63.2% of tumors with vascular invasion showed CD24 expression (p=0.006)(Table 1). These results suggest that the positive expression of CD24 characterizes gastric carcinomas with more aggressive phenotype.
It is well known that the depth of tumor invasion, distant metastasis and lymph node metastasis are the major prognostic factors in gastric carcinoma. Recently, the expression of different cell surface antigens in various malignant tumors has been reported to be one of the biologic markers of malignant potential. By subtractive technique, CD24 was selected as one of metastasis-associated genes, and CD24 was confirmed to be overexpressed in the metastatic phenotype (9). The mechanism of cancer progression resulting from expressional alteration remains unknown. However, CD24 overexpression may be useful as a marker that indicates invasiveness and poor prognosis.
The mechanism of GPI-anchored protein-mediated signaling remains intriguing, because these proteins do not have any cytoplasmic domain to transduce intracellular signals. A recently proposed concept of glycolipid-enriched membrane (GEM) domains or rafts is expected to provide an explanation for the signaling properties of GPI-anchored proteins (10). CD24 molecules are distributed only in the GEM fractions, and CD24 mediates a negative signal for B cell survival through a GEM-dependent mechanism, that is closely related to the B cell receptor (BCR)-mediated apoptotic signal. This in turn suggests that CD24 mediates the apoptotic signal in B cells upon ligand stimulation or that CD24-mediated stimuli augment BCR- mediated apoptosis in Burkitt's lymphoma cells (11).
It has been speculated that human tumors may use selectins or their ligands during invasion and metastasis (1). In fact, inflammation and trauma, which induce the expression of selectins, can influence the spread of tumor cells. The CD24/P-selectin binding pathway could be important in the dissemination of tumor cells by facilitating the interaction with platelets or endothelial cells (12). The function of CD44 is principally dependant on cellular adhesion. In addition, the CD44 receptor and E-selectin are currently potential candidates for involvement in the extravasation process of gastric tumor cells, which includes the lodging of malignant cells between endothelial cells and their subsequent adhesion to the extracellular matrices, leading to the manifestation of metastatic nodules (13).
Furthermore, it has been found that CD24 can act as a specific ligand for P-selectin (1) and that CD24 expressed by breast carcinoma is necessary and sufficient to support P-selectin dependent rolling in vitro and in vivo (14). Taken together, these findings may indicate that both CD24 and CD44 contribute to the process of metastasis simultaneously through binding with P-selectin and E-selectin, respectively. This is supported by the fact that each of three selectins can have unique binding sites coexisting on a given carcinoma mucin molecule (15). Such a theory supports our results, wherein CD24 positive expression was observed in 63% of the cases of carcinoma with vascular invasion (p=0.006), and increased positive expression of CD44 was observed in the CD24 positive cases.
PTEN is a multifunctional protein endowed with a phosphatase activity capable of dephosphorylating not only proteins, but also phospholipids of the phosphatidylinositol pathway. Its protein phosphatase activity allows it to affect the interactions of cells with the intercellular matrix. These interactions are important in the mechanism of invasion (16). Thus, PTEN can inhibit cell growth, invasion, migration, and focal adhesions. It has been reported that the silencing of the PTEN gene occurs frequently in gastric carcinoma, and that the loss of expression of the PTEN gene is associated with tumor progression, metastasis and poor survival (8,17). It is interesting to note that the PTEN+/CD24- group showed the best prognosis compared to other groups. The clinical relevance of this finding should be confirmed by further experiments.