AbstractHypoxia plays a major role in the induction of angiogenesis during tumor development. One mechanism by which tumor cells respond to a reduced oxygen level is via the activation of hypoxia-inducible factor-1 (HIF-1). HIF-1 is an oxygen-dependent transcriptional activator that plays crucial roles in the angiogenesis of tumors and mammalian development. HIF-1 consists of a constitutively expressed HIF-1β subunit and the highly regulated HIF-1α subunits. The stability and activity of HIF-1α are regulated by various post-translational modifications, hydroxylation, acetylation, phosphorylation and sumoyaltion. Therefore, HIF-1α interacts with several protein factors including PHD, pVHL, ARD-1, SUMO and p300/CBP. Under normoxia, the HIF-1α subunit is rapidly degraded via the von Hippel-Lindau tumor suppressor gene product (pVHL)-mediated ubiquitin/proteasome pathway. The association of pVHL and HIF-1α under normoxic conditions is triggered by the hydroxylation of prolines and the acetylation of lysine within a polypeptide segment known as the oxygen-dependent degradation (ODD) domain. On the contrary, under the hypoxia condition, the HIF-1α subunit becomes stable and interacts with coactivators such as p300/CBP to modulate its transcriptional activity. Under hypoxic conditions, HIF-1 eventually acts as a master regulator of numerous hypoxia-inducible genes. The target genes of HIF-1 are especially related to angiogenesis, cell proliferation and survival, and to glucose and iron metabolism. Moreover, it was reported that the activation of HIF-1α is closely associated with a variety of tumors and oncogenic pathways. Hence, the blocking of HIF-1α itself or the blocking of HIF-1α interacting proteins inhibits tumor growth. Based on these findings, HIF-1 can be a prime target for anticancer therapies. Therefore, this review summarizes the molecular mechanism of HIF-1α stability, the biological functions of HIF-1 and its potential applications for cancer therapies.
INTRODUCTIONHypoxia is a reduction in the normal level of tissue oxygen tension, and it occurs during several pathophysiological processes including tumorigenesis. Hypoxia may occur during the initial avascular phase or it may develop in established tumors as a result of new blood vessels formation that is ineffective and provides only poor blood flow. Hence, the growth of tumors is restricted by limited oxygen and nutrients when they are too distant from any nearby vessels. In order to overcome these restrictions, the tumors acquire the ability to recruit their own blood supply system under hypoxic stress (1). Although hypoxia generates an unfavorable situation for cell growth, cancer cell undergo a series of genetic and metabolic changes that allow them to survive and even proliferate.
The hypoxia-inducible factor-1 (HIF-1) is the most important factor involved in the cellular response to hypoxia, and it has been extensively studied during this last decade (2,3). In this review, we will summarize the oxygen dependent regulation of HIF-1 stability and function, and also its chemical implication in the field of anticancer therapies.
Hypoxia-inducible Factor (HIF); Ringmaster of the Hypoxic World1) Structure of HIF-1HIF-1 has been shown to be a heterodimeric consisting of an α subunit and a β subunit, and a β subunit is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1α is the subunit that is highly regulated by the oxygen level, but HIF-1β is the constitutively expressed subunit (4). Each subunit has the basic helix-loop-helix (bHLH) and the PER-ARNT-SIM (PAS) domain. The N-terminal half of HIF-1 α contains the bHLH and PAS domains that are required for dimerization and DNA binding (5). The C-terminal half contains the domains that are required for degradation and transactivation: the oxygen-dependent degradation (ODD) domain, which confers oxygen dependent instability, two independent transactivation domains (N-TAD and C-TAD), and an inhibitory domain (ID) that negatively regulates the transactivation domains (6). Whereas the N-terminal TAD (N-TAD) also constitutes a degradation box, the C-terminal TAD (C-TAD) functions in a strictly hypoxia-inducible fashion. The C-TAD interacts with a coactivator such as p300/CBP, it is independent of protein stability and is required for full HIF activity (7) (Fig. 1).
2) HIF-α isoformsHomology searches in the gene bank and cloning experiments have found other members of bHLH-PAS superfamily: HIF-2α, which is referred to as endothelial PAS domain protein 1 (EPAS1), and also HIF-3α (8). These isoforms have structural similarity and are classified as members of the bHLH-PAS family. The structure and functions of HIF-1α have been more extensively studied than those of HIF-2α or HIF-3α. Six isoforms of HIF-1α have been reported. HIF-1αFL is similar to the wild type HIF-1α except for an additional three base pairs, TAG, that are between exon 1 and exon 2. Next, HIF-1α736 loses exon 14, and so it lacks the C-TAD. HIF-1αFL and HIF-1α736 activate the VEGF promoter upon hypoxia (9). In contrast to HIF-1αFL and HIF-1α736, HIF-1α557 and HIF-1α516 function as the dominant negative isoforms of HIF-1α. HIF-1α557 loses exon 12, which is induced by the zinc ion, and HIF-1α516 lacks exon 11 and 12 (10,11). Recent studies have reported that the isoform HIF-1α785 contains all the functional domains; therefore, HIF-1α785 acts as a transcriptional activator, but it lacks exon11 in the ODD domain (12). Another HIF-1α variant, HIF-1α417, conserves only the bHLH and PAS domains, which are essential for the binding between DNA and ARNT. However it lacks all the other important domains of HIF-1α, such as the ODD domain and TAD (13). Not much detail is known about the roles of the 2α and 3α class subunits. Like HIF-1α, HIF-2α is also regulated by enzymatic hydroxylation of the conserved proline residue that causes its degradation under normoxia conditions via the ubiquitin E3 ligase complex (14~17). It has recently been reported that HIF-3α also undergoes degradation through the polyubiquitination-proteasome pathway (18). Human HIF-3α has multiple spliced variants: HIF-3α1~6 (Fig. 2). HIF-3α1, 2 and 3 share a common ODD that includes the consensus motif of proline hydroxylase and it binds the pVHL E3 ligase complex under normoxic conditions. Dominant negative regulator of HIF-α function, IPAS, is generated by alternative splicing of the HIF-3α locus (19). IPAS prevents the interaction of HIF-1α with HIF-1β because IPAS dimerizes HIF-1α, and the IPAS/HIF-1α complex does not bind to the hypoxia-response element. So it inhibits transcriptional activation of HIF-1α. In contrast to HIF-3α, HIF-2α activates transcription and induces the hypoxia-mediated gene expression of such factors as VEGF. It was reported that hypoxia did not affect the mRNA levels of HIF-1α, HIF-1β and HIF-2α, but HIF-3α mRNA was increased after 2 hr of hypoxia (20). However, how the hypoxia increases the mRNA level of HIF-3α is not known. Therefore, further study concerning the expression and stability of HIF-3α is required.
The effect of the HIF family on gene expression may be different according to the cell types. For example, HIF-1α and -2α are abundantly expressed in the kidney. Nevertheless, the overexpression of HIF-2α, but not HIF-1α, promotes the growth of renal carcinoma cells (21~23). However, in the breast cancer cell line, HIF-1α is the major isoform required for the induction of hypoxic genes (24).
3) HIF-1 target genesHIF-1 activity leads to the upregulation of genes that are involved in many aspects of cancer progression, angiogenesis, cell survival, glucose metabolism and invasion. More than 60 putative HIF-1 target genes have currently been identified (Fig. 3). HIF-1 targets the genes that are particularly relevant to cancer, and these genes encode angiogenic factors, glucose transporters and glycolytic enzymes, survival factor and invasion factor (25).
(1) AngiogenesisVascular endothelial cell growth factor, (VEGF), is the most potent endothelial-specific mitogen to directly participate in angiogenesis (26~29), and it is one of the major target genes for HIF-1. This growth factor interacts with its receptor, VEGFR, which is specifically expressed in endothelial cells, and this stimulates endothelial cell proliferation (27,28,30,31). It has also been shown that hypoxia induces the expression of VEGF mRNA and protein, suggesting that hypoxia is a stimulus for angiogenesis through the up-regulation of VEGF expression (27,28,30,31). The induction of angiogenesis leads to an increase in the vascular density and hence, a decrease in the oxygen diffusion distance. However, local blood flow under pathophysiological conditions is controlled by modulation of the vascular tone through the production of NO (inducible nitric oxide synthase), CO (heme oxygenease 1), endothelin 1, adrenomedulin or the activation of the α1B-adrenergic receptor, and all of theseinvolve the HIF-1 target genes (32~37). Therefore, HIF-1 contributes to angiogenesis by far more complex mechanisms than simple VEGF induction, and HIF-1 probably works by recruiting additional target genes that are involved in vessel maturation (38).
(2) Growth/survivalHypoxia-induced growth factors are known to promote cell proliferation and survival. Several growth factors, most notably insulin-like growth factor-2 (IGF2) and transforming growth factor (TGF)-α, are also HIF-1 target genes (39,40). Binding of these factors to their cognate receptors-the insulin-like growth factor 1 receptor (IGFIR) and epidermal growth factor receptor (EGFR), respectively-activates signal transduction pathways that lead both to HIF-1α expression and to cell proliferation and survival (3). The p42/p44 mitogen-activated protein kinases, which regulate cell proliferation in response to extra-cellular growth factors, have been shown to phosphorylate HIF-1α and activate transcription of HIF-1 target genes (27). Phosphatidylinositol 3-OH kinase (PI3K) activity is also increased in some cell types under hypoxic conditions (41). PI3K is one of the key downstream mediators of many tyrosine kinase signaling pathways, and it is involved in regulating cell proliferation and suppression of apoptosis. The PI3K pathway is inhibited by the phosphoinositide phosphatase PTEN, and mutations in PTEN enhance HIF-1 activated responses (42). PTEN regulates cell growth and proliferation, and it is deleted or mutated in several human cancers, including glioblastoma, endometrial tumors and prostate cancer (43). Therefore, HIF-1 contributes to the autocrine-signaling pathways that are crucial for cancer progression.
(3) Glucose metabolismDepletion of oxygen changes the energy metabolism of cells: the cells don't use the oxygen-dependent metabolic pathway such as the TCA cycle. Instead of the TCA cycle, the cells switch to the oxygen-independent metabolic pathway, and they start using glycolysis as the primary mechanism of ATP production (44,45). The TCA cycle provides 38 ATPs from glucose, but glycolysis provides only two. Therefore, the hypoxic condition requires more glycolysis than normoxic condition. Many genes involved in glucose uptake and glycolysis have been identified as HIF-1 target genes (46). HIF-1 regulates the expression of all enzymes in the glycolytic pathway, as well as expression of the glucose transporters GLUT1 and GLUT3 that mediate cellular glucose uptake (47). Enhanced lactate production and hence, a decrease in pH, results from the increase in anaerobic glycolysis, and this potentially limits this source of ATP despite a sufficient glucose supply. Transmembrane carbonic anhydrases were reported to be HIF-1 target genes (48), and in fact, increased glycolysis is a normal response to proliferation: moreover, migrating cells also use this pathway as an energy source (49). The intermediary metabolites of the glycolytic pathway provide the precursors for synthesis of glycine, serine, purine, pyrimidine and phospholipids, all of which are essential for cell growth and the maintenance of cells that are under stressful conditions (43).
(4) Invasion and metastasisHypoxia unleashes the invasive and metastatic potential of tumor cells. HIF-1 regulates the expression of genes encoding cathepsin D, matrix metalloproteinase 2, urokinase plasminogen activator receptor (uPAR), fibronectin 1, keratins 14, 18 and 19, vimentin, transforming growth factor alpha, and autocrine motility factor, and these are all proteins that play established roles in the pathophysiology of invasion (3,39).
Molecular mechanism of HIF-1α stabilityThe first regulator of HIF-1 is oxygen, and HIF-1α appears to be the specific HIF-1 subunit regulated by hypoxia. HIF-1α protein is subject to rapid degradation at normoxia by the process of the pVHL-mediated ubiquitin-proteasome pathway, whereas hypoxia blocks this degradation and leading to accumulation of HIF-1α protein (50,51). The association of HIF-1α with pVHL is triggered by the post-translational hydroxylation of a proline residue that is mediated by prolyl hydroxylase (PHD) or HIF prolyl hydroxylase (HPH). The HIF-1α contains two sites for hydroxylation, Pro 402 and Pro 564, within its ODD domain and each site contains a conserved LXXLAP motif (15). The hydroxyproline residue becomes buried within the hydrophobic core of pVHL, the von Hippel-Lindau tumor suppressor protein, which is a part of the ubiquitin ligase protein complex (52,53).
All three paralogs of the HPH/PHD family have been discovered and named as follows: HPH-1/PHD-3, HPH-2/PHD-2 and HPH-3/PHD-1. When overexpressed as a tagged protein, HPH-2/PHD-2 resides in the cytoplasm, whereas HPH-3/PHD-1 is predominantly observed in the nucleus (54,55). Berra et al. (2003) proposed that HPH-2/PHD-2 is primarily responsible for HIF hydroxylation in the normoxic condition.
The prolyl hydroxylase is dioxygenase requiring oxygen and 2-oxoglutarate as a substrate. This enzyme transfers one oxygen atom to the proline residue, and the second oxygen atom reacts with 2-oxoglutarate to generate succinate. The activity of PHD to HIF-1α is known to depend on the O2 concentration. Therefore, PHD has been suggested to be an oxygen sensor (56,57).
Another protein interacting with the ODD domain of HIF-1α is ARD1 acetyltransferase (58). The yeast homolog of ARD-1 is required for the expression of protein N-acetyltransferase (NAT) in lower eukaryotes and bacteria, but the function of NAT has not been defined in mammalian cells (59,60). Jeong et al. (2002) reported that ARD1 acetylates the Lys-532 residue in the ODD domain of HIF-1α by transferring an acetyl group from Ac-CoA. The acetylation level of HIF-1α gradually decreases as the length of hypoxic exposure time increases, which is due to the reduced expression of ARD1. The acetylation of Lys532 by ARD1 is critical to the proteasomal degradation of HIF-1α. A K532R mutant that was not acetylated by ARD1 was stabilized and it showed a decreased interaction with pVHL (58). It is interesting that Lys532 was previously reported as being critical for the degradation of HIF-1α under the normoxic condition (12,61). Although it is unclear how the acetylation of HIF-1α leads to its decreased stability, a conformational change of HIF-1α may effectively increase its interaction with pVHL and enhance the subsequent proteasomal degradation.
HIF-1α is also sumoylated by SUMO-1, which is the small ubiquitin-like protein family. This modification leads to an increase in HIF-1α stability and an increase in its transcriptional activity, implying that SUMO-1 may counteract ubiquitin. We recently reported that sumoylation occurs at Lys391/Lys477 residues, but not at the Lys532 residue (Fig. 4)(62)
Overexpression of HIF-1α in the tumorImmunohistochemical analysis of human tumor biopsy specimens has revealed the dramatic overexpression of HIF-1α in common cancers (63,64). This is a result of intratumoral hypoxia, genetic alteration and the increased HIF-1α transcriptional activity. In addition to hypoxia, HIF accumulation may occurs as a result of genetic alteration such as the loss or the simple decrease of pVHL (31). Other known causes of increased HIF-1α transcriptional activity include the activation of mitogen-activated protein kinase (65) and the insulin-like growth factor 1 pathway (40,42). In any case, the activated HIF pathway triggers biologic events that are intimately associated with aggressive tumor behavior. Thus, HIF-1α overexpression may be a marker of highly aggressive disease behavior for several different tumor types (Table 1). HIF-1α expression in tumor specimens has been analyzed by immunohistochemistry for cancers of the brain (oligodendroglioma), breast, cervix, melanoma, oropharynx, ovary pancreas, prostate and uterus (endometrial).
HIF-1 targeted therapiesBecause tumor cell invasion and migration are reinforced by hypoxic stimuli, hypoxia is a major obstacle for tumor radiotherapy, and it is often a problem in chemotherapy too. HIF-1α-expressing tumors are expected to be resistant to radiation therapy because these kinds of factors denote tumor hypoxia and an enhanced transcription of proteins that will favor tumor cell survival (96). Thus, blocking of HIF-1α activity may be advantageous for inhibiting cancer progression as this would help starve the growing tumors of their oxygen and nutrient supply (66). Recent studies have provided evidence indicating that HIF-1α mediates resistance to chemotherapy and radiation (67,68). Inhibition of HIF-1α activity could, therefore, represent an important component of a combination anti-angiogenic therapy, and strategies for blocking HIF-1α itself or the HIF-1α interacting proteins are under development. HIF-1α antisense therapy might act synergistically with the appropriate immunotherapy. In vivo delivery of antisense to HIF-1α alone by a direct intratumor injection was shown to inhibit tumor growth, but a combination of the two treatments caused marked tumor regression and a sustained antitumor immune response (69). A gene-therapy strategy to block the interaction between HIF-1α and its transcriptional co-activator CBP/p300 led to the attenuation of hypoxia-inducible gene expression and the inhibition of tumor growth in a mouse xenograft model (70). In addition, HIF-1α interacts with the chaperone HSP90, and the HSP90 inhibitor 17-allyl-aminogel-danamycin (17-AAG) induces HIF-1α degradation in a VHL-independent manner (71~73). The small molecule YC-1 (3-(-5'-hydroxy-methyl-2'-furyl)-1-benzylindazole) was also shown to reduce both the HIF-1α levels and xenograft growth (74). The mechanism by which YC-1 reduces HIF-1α levels has not been established, although YC-1 is known to stimulate soluble guanylate-cyclase activity, yet this effect is not required for inhibiting HIF-1α levels. Disruption of microtubule polymerization by 2-methoxyestradiol (2ME2) has also been shown to result in decreased HIF-1α levels (Table 2) (75).
Hypoxia response elements (HREs) that are linked to marker genes or prodrug activation systems can be used to selectively activate therapeutics in hypoxic regions (76,77). Gene-therapy vectors that carry pro-apoptotic or anti-proliferation genes driven by HREs can be selectively targeted to cancer cells in hypoxic regions of the tumor (31). For example, in vivo, HRE-mediated trans gene expression was localized adjacent to areas of pyknotic cells and necrosis (76). In addition to the anti-angiogenesis agents, it is clear that many novel therapeutic agents targeting signal-transduction pathways have anti-angiogenic effects. This effect seems to be due in part to the fact that inhibition of the signal-transduction pathways results in decreased levels of HIF-1α (3).
CONCLUSIONSHypoxia is a common physiological feature of all tumors, and HIF-1α is a master regulator among a lot of different transcription factors and functions that depend on oxygen tension. Therefore, HIF-1α stabilization is critical for those events that are mediated by hypoxia and dominated by post-translational modification.
Hydroxylation and acetylation are essential to the regulation of HIF-1α protein stability. Under normoxic conditions, the HIF-1α ODD domain encompasses several sequences that mediate O2-dependent ubiquitination of HIF-1α protein through an interaction with pVHL, which is an E3 ubiquitin-protein ligase that targets HIF-1α for proteasomal degradation (58,78~81). Furthermore, ubiquitination of HIF-1α is mediated by an interaction with p53, and this promotes Mdm-2-mediated ubiquitination and proteasomal degradation of HIF-1α through direct interaction with HIF-1α during hypoxia (82,83). Under hypoxia conditions, HIF-1α is stabilized, which is determined by a balance between negative regulators such as p53 and positive unknown regulatory factors, and HIF-1α is then accumulated in the nucleus (84). Stabilized HIF-1α exerts its transcriptional activity by binding to the p300/CBP, the SRC (steroid receptor coactivator-1) family coactivators, nuclear redox regulator Ref-1 and the molecular chaperon heat shock protein 90 (HSP 90) (84~87). They all synergistically enhance HIF-1α-mediated transcriptional regulation under hypoxic conditions. The modulation of HIF-1α stability and its activation requires the interaction of these multiproteins with HIF-1α (88,113,114).
We recently reported that sumoylation increased HIF-1α stability (62). Sumoylation on Lys391 and Lys477 in the ODD domain may increase HIF-1α stability by competing with hydroxylation and acetylation for the pVHL binding.
In addition to angiogenesis, HIF-1α is also a master transcription factor related to cell proliferation and survival, glucose metabolism and iron metabolism. Therefore, HIF-1α is a major player in of the many diseases that generate a hypoxic microenvironment such as cancer, stroke, and heart disease (89). Especially, hypoxic tumor conditions might activate the expression of genes that promote tumor growth, and this would lead to a more aggressive tumor phenotype (43). Thus, the activation of HIF-1α has been associated with a variety of tumors and oncogenic pathways. On the contrary, the blocking of HIF-1α itself or the blocking of HIF-1α interacting proteins inhibits tumor growth (3). HIF-1α antisense therapy is a gene-therapy strategy to block the interaction between HIF-1α and its transcriptional co-activator CBP/p300 and other small molecules such as 17AAG and YC-1, and it has displayed a good possibility as a cancer therapy (69,70). Based on these findings, HIF-1 can be a prime target for anticancer therapies (3,43), and therefore, increased understanding of HIF-1α regulation will provide the cornerstone for novel therapeutic approaches.
NotesThis work was supported by the Creative Research Initiatives Program of the Ministry of Science and Technology, the 21C Frontier R&D Program (FG04-21-01), the Ministry of Science and Technology, and the Korea Health 21 R&D Project (HMP-01-PJ1-PG1-01CH04-0005), Korea. References1. Cameliet P, Jain RK. Angiogenesis in cancer and other disease. Nature. 2000;407:249–257. PMID: 11001068
2. Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, et al. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004;68:971–980. PMID: 15313390
4. Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1999;22:172–178. PMID: 1848205
5. Wang GL, Jiang BH, Semenza GL. Effect of protein kinase and phosphatase inhibitors on expression of HIF-1. Biochem Biophys Res Commun. 1995;216:669–675. PMID: 7488163
6. Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of HIF-1α. J Biol Chem. 1996;271:17771–17778. PMID: 8663540
7. Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002;277:38723–38730. PMID: 12133832
8. Tian H, Mcknight SL, Russel DW. Endothelial PAS domain protein 1, a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. PMID: 9000051
9. Gothie E, Richard DE, Berra E, Pages G, Pouyssegur J. Identification of alternative spliced variants of human hypoxic-inducible factor-1alpha. J Biol Chem. 2000;275:6922–6927. PMID: 10702253
10. Chun YS, Choi EJ, Yeo EJ, Lee JH, Kim MS, Park JW. A new HIF-1 alpha variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J Cell Sci. 2001;114:4051–4061. PMID: 11739637
11. Chun YS, Choi EJ, Kim TY, Kim MS, Park JW. A dominant-negative isoform lacking exon 11 and 12 of the human hypoxic-inducible factor-1α gene. Biochem J. 2002;362:71–79. PMID: 11829741
12. Chun YS, Lee KH, Choi EJ, Bae SY, Yeo EJ, Huang LE, et al. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1α isoform: implications for tumor promotion. Cancer Res. 2003;63:8700–8707. PMID: 14695184
13. Lee KH, Park JW, Chun YS. Non-hypoxic transcriptional activation of the aryl hydrocarbon receptor nuclear translocator in concert with a novel hypoxia-inducible factor-1alpha isoform. Nucleic Acids Res. 2004;32:5499–5511. PMID: 15479785
14. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. PMID: 11292862
15. Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. PMID: 11566883
16. Maxwell P, Weisner M, Chang GW, Clifford S, Vaux E, Pugh C, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. PMID: 10353251
17. Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia inducible factor-1 alpha by the von Hipple-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. PMID: 10944113
18. Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, et al. Multiple splice variants of the human HIF-3alpha locus are targets of the VHL E3 ubiquitin ligase complex. J Biol Chem. 2003;278:11032–11040. PMID: 12538644
19. Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J Biol Chem. 2002;277:32405–32408. PMID: 12119283
20. Heidbreder M, Frohlich F, Johren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3α mRNA expression. FASEB J. 2003;17:1541–1543. PMID: 12824304
21. Rosenberger C, Mandriota S, Jurgensen JS, Wiesner MS, Horstrup JH, Frei U, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1974–1976. PMID: 12089396
22. Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF-1alphaz to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. PMID: 12086861
23. Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG. Inhibition of HIF is necessary for tumor suppression by the von Hippel Lindau protein. Cancer Cell. 2002;1:237–246. PMID: 12086860
24. Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2002;60:7106–7113. PMID: 11156418
25. Berta M, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. Hypoxia-inducible factor-1a is modified by covalent attachment to SUMO. Keystone Symposia Conference: Biology of Hypoxia: The Role of Oxygen Sensing in Development, Normal Function and Disease. 2004.
26. An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000;126:153–160. PMID: 10741909
27. Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000;19:139–145. PMID: 11191053
28. Josko J, Gwozdz B, Jedrzejowska-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. PMID: 11208453
29. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. PMID: 11166264
30. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. PMID: 9872925
31. Harris AL. von Hippel-Lindau syndrome: target for anti-vascular endothelial growth factor (VEGF) receptor therapy. Oncologist. 2000;5(Suppl.1):32–36. PMID: 10804089
32. Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. PMID: 7500013
33. Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274:L212–L219. PMID: 9486205
34. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. PMID: 9038135
35. Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. PMID: 9588211
36. Nguyen SV, Claycomb WC. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem Biophys Res Commun. 1999;265:382–386. PMID: 10558876
37. Eckhart AD, Yang N, Xin X, Faber JE. Characterization of the alpha1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA. 1997;94:9487–9492. PMID: 9256509
38. Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. PMID: 12153983
39. Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. PMID: 12615733
40. Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. PMID: 10463582
41. Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. PMID: 11289110
42. Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. PMID: 10691731
43. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. PMID: 11902584
44. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. PMID: 10098401
45. Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21:3436–3444. PMID: 11313469
46. Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. PMID: 10729275
47. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. PMID: 11120745
48. Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. PMID: 11156414
49. Mazurek S, Boschek CB, Eigenbrodt E. The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1997;29:315–330. PMID: 9387092
50. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of HIF depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. PMID: 8943284
51. Kallio PJ, Pongratz I, Gradin K, Mcguire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94:5667–5672. PMID: 9159130
52. Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417:975–978. PMID: 12050673
53. Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP. Structure of an HIF-1α-pVHL complex: Hydroxyproline recognition in signalling. Science. 2002;296:1886–1889. PMID: 12004076
54. Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia inducible factor-1α for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. PMID: 12181324
55. Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I. Intracellular localization of human HIF-1α hydroxylases: Implications for oxygen sensing. J Cell Sci. 2003;116:1319–1326. PMID: 12615973
56. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. PMID: 11595184
57. Jewell UR, Kveitikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of Hif-1alpha in response to hypoxia is instantaneous. FASEB J. 2001;15:1312–1314. PMID: 11344124
58. Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. PMID: 12464182
59. Tribioli C, Mancini M, Plassart E, Bione S, Rivella S, Sala C, et al. Isolation of new genes in distal Xq28: transcriptional map and identification of a human holologue of the ARD1 N-acetyl transferase of Saccharomyces cerevisiae. Human Mol Genet. 1994;3:1061–1067. PMID: 7981673
60. Ingram AK, Cross GA, Horn D. Genetic manipulation indicates that ARD1 is an essential N-acetyltransferase in Trypansoma brucei. Mol Biochem Parasitol. 2000;111:309–317. PMID: 11163439
61. Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. PMID: 10944113
62. Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, et al. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004;324:394–400. PMID: 15465032
63. Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. PMID: 10934146
64. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. PMID: 10582706
65. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. PMID: 9345014
66. Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–790. PMID: 12603311
67. Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. PMID: 12761491
68. Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. PMID: 11306467
69. Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001;8:638–645. PMID: 11320410
70. Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. PMID: 11100117
71. Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. PMID: 12052835
72. Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteasome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. PMID: 11980636
73. Zagzag D, Nomura M, Friedlander DR, Blanco CY, Gagner JP, Nomura N, et al. Geldanamycin inhibits migration of glioma cells in vitro: a potential role for hypoxia-inducible factor (HIF-1alpha) in glioma cell invasion. J Cell Physiol. 2003;196:394–402. PMID: 12811834
74. Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. PMID: 12671019
75. Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. PMID: 12726862
76. Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. PMID: 9142119
77. Lemmon MJ, van Zijl P, Fox ME, Mauchline ML, Giaccia AJ, Minton NP, et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997;4:791–796. PMID: 9338007
78. Sutter CH, Laughner E, Semenza GL. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA. 2000;97:4748–4753. PMID: 10758161
79. Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. PMID: 10823831
80. Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA. 2000;97:10430–10435. PMID: 10973499
81. Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. PMID: 9278421
82. Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. PMID: 10640274
83. An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. PMID: 9537326
84. Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. PMID: 10202154
85. Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. PMID: 8917528
86. Carrero P, Okamoto K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol Cell Biol. 2000;20:402–415. PMID: 10594042
87. Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, et al. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. PMID: 10544245
88. Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, et al. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002;277:9–12. PMID: 11707426
89. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. PMID: 10749844
90. Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–1837. PMID: 12060624
91. Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:494–501. PMID: 12738326
92. Birner P, Gatterbauer B, Oberhuber G, Schindl M, Rossler K, Prodinger A, et al. Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer. 2001;92:165–171. PMID: 11443623
93. Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. PMID: 11556841
94. Takahashi R, Tanaka S, Hiyama T, Ito M, Kitadai Y, Sumii M, et al. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797–802. PMID: 12792726
95. Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. PMID: 11980639
96. Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piazza M, et al. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001;61:1830–1832. PMID: 11280732
97. Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–225. PMID: 11566613
98. Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. PMID: 12154035
99. Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. PMID: 11245461
100. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. PMID: 10678579
101. Koukourakis MI, Simopoulos C, Polychronidis A, Perente S, Botaitis S, Giatromanolaki A, et al. The effect of trastuzumab/docatexel combination on breast cancer angiogenesis: dichotomus effect predictable by the HIFI alpha/VEGF pretreatment status? Anticancer Res. 2003;23:1673–1680. PMID: 12820439
102. Fujiwara K, Kiura K, Ueoka H, Tabata M, Hamasaki S, Tanimoto M. Dramatic effect of ZD1839 ('Iressa') in a patient with advanced non-small-cell lung cancer and poor performance status. Lung Cancer. 2003;40:73–76. PMID: 12660009
103. Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–50086. PMID: 12401798
104. Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. PMID: 10551817
105. Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. PMID: 12657718
106. Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol. 2002;62:975–982. PMID: 12391259
107. Kurebayashi J, Otsuki T, Kurosumi M, Soga S, Akinaga S, Sonoo H. A radicicol derivative, KF58333, inhibits expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Jpn J Cancer Res. 2001;92:1342–1351. PMID: 11749701
108. Kubo T, Maezawa N, Osada M, Katsumura S, Funae Y, Imaoka S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1alpha (HIF-1alpha): structural requirement of bisphenol A for degradation of HIF-1alpha. Biochem Biophys Res Commun. 2004;318:1006–1011. PMID: 15147973
109. Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG. Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol. 2004;66:612–619. PMID: 15322253
110. Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3:233–244. PMID: 15026543
111. Chang H, Shyu KG, Lee CC, Tsai SC, Wang BW, Hsien Lee Y, et al. GL331 inhibits HIF-1alpha expression in a lung cancer model. Biochem Biophys Res Commun. 2003;302:95–100. PMID: 12593853
112. Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. PMID: 9782081
113. Moon EJ, Jeong CH, Jeong JW, Kim DR, Yu DY, Murakanim S, et al. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1α. FASEB J. 2004;18:382–384. PMID: 14688211
114. Bae MK, Jeong JW, Kim SH, Kim SY, Kang HJ, Trentin GA, et al. Tid-1 interacts with pVHL and modulates angiogenesis by destabilization of HIF-1α. Cancer res. (accepted)
Fig. 1Domain structures of HIF-1α and their potential function. HIF-1α possesses the basic helix-loop-helix (bHLH) and PER-ARNT-SIM (PAS) domains that are involved in dimerization with HIF-1β and DNA binding. Its C-terminal part contains two transacting domains (TAD) and an inhibitory domain (ID). The N-TAD lies within its ODD domain. The ODD domain regulates the stability of HIF-1α via recognition by the von Hippel-Lindau E3 ubiquitin ligase (pVHL). 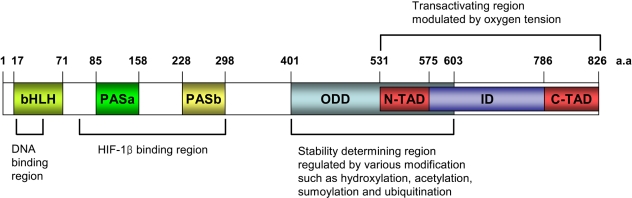
Fig. 2Splice variants of the HIF α subunit. bHLH: basic helix-loop-helix, PAS: Per/Arnt/Slim domain, ODD: oxygen dependent degradation domain, N-TAD: N-terminal transactivation domain, C-TAD: C-terminal transactivation domain, LZIP: leucine zipper. 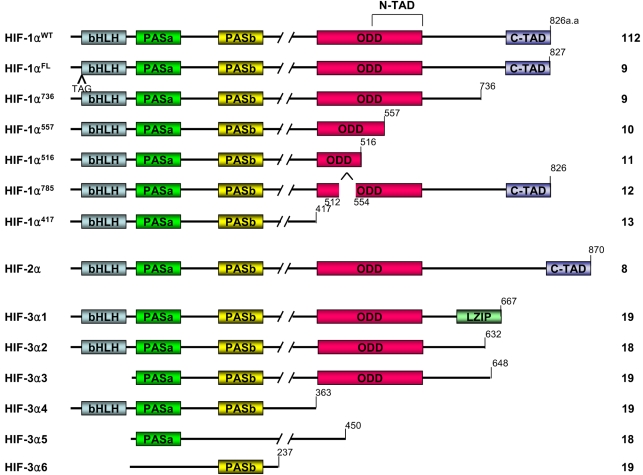
Fig. 3Target genes that are transcriptionally activated by HIF-1α1B-AR: α1B-adrenergic receptor, ADM: adrenomedullin, AK3: adenylate kinase 3, ALDA: aldolase A, ALDC: aldolase C, AMF: autocrine motility factor, CA9: Carbonic anhydrase 9, CATHD: cathepsin D, EG-VEGF: endocrine-gland-derived VEGF, ENG: endoglin, ET1: endothelin-1, ENO1: enolase 1, EPO: erythropoietin, FN1: fibronectin 1, GLUT1: glucose transporter 1, GLUT3: glucose transporter 3, GAPDH: glyceraldehyde-3-P-dehydrogenase, HK1: hexokinase 1, HK2: hexokinase 2, IGF2: insulin-like growth-factor 2, IGF-BP1: IGF-factor-binding-protein 1, IGF-BP2: IGF-factor-binding-protein 2, IGF-BP3: IGF-factor-binding-protein 3, ITF: intestinal trefoil factor, KRT14: keratin 14, KRT18: keratin 18, KRT19: keratin 19, LDHA: lactate dehydrogenase A, LEP: leptin, LRP1: LDL-receptor-related protein 1, MDR1: multidrug resistance 1, MMP2: matrix metalloproteinase 2, NOS2: nitric oxide synthase 2, PFKBF3: 6-phosphofructo-2-kinase/fructose-2:6-biphosphatase-3, PFKL: phosphor-fructo kinase L, PGK 1: phosphoglycerate kinase 1, PAI1: plasminogen-activator inhibitor 1, PKM: pyruvate kinase M, TGF-α: transforming growth factor-α, TGF-β3: transforming growth factor-β3, TPI: triosephosphate isomerase, VEGF: vascular endothelial growth factor, UPAR: urokinase plasminogen activator receptor, VEGFR2: VEGF receptor-2, VIM: vimentin. 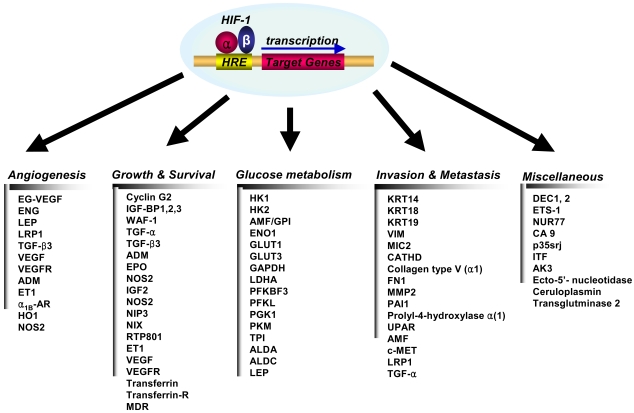
Fig. 4Molecular mechanism of HIF-1α stability. HIF-1α is subject to rapid degradation at normoxia by the pVHL-meditated ubiquitin-proteasome pathway, whereas hypoxia blocks degradation of HIF-1α leads to HIF-1α accumulation. HIF-1α hydroxylation on Pro402 and Pro564 and acetylation on Lys532 within the ODD domain facilitates binding with pVHL. As a result, HIF-1α is degraded via the ubiquitin-proteasome pathway. By contrast, sumoylation on Lys391 and Lys477 in the ODD domain may increase HIF-1α stability by competing with hydroxylation and acetylation for the pVHL binding. 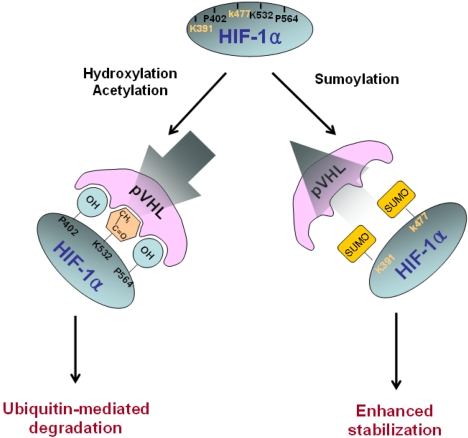
|
|
||||||||||||||||||||||||||||||||||||||