INTRODUCTION
Gastric cancer is the most common carcinoma in Korea (1) but it is often diagnosed in the locally advanced or metastatic stage (2). In the past three decades, several chemotherapy regimens have been developed for the treatment of advanced gastric cancer (3). Systemic chemotherapy is a widely accepted palliative treatment, which may lead to an objective response, an improvement in the quality of life, and prolonged survival. However, more than 50% of patients with advanced gastric cancer who receive chemotherapy fail to respond, and even in those that do, the duration of the response is short-lived, less than 10 months (3). Moreover, advanced gastric cancer patients who fail to respond or who progress after the first-line chemotherapy have a poor prognosis. Accordingly, various second-line regimens have been investigated but the results have remained unsatisfactory; the response rates rarely exceed 20%, and there are many side effects (4~6). Therefore, there is a need for new treatments with better therapeutic indices in addition to novel agents with lower levels of cross-resistance.
Oxaliplatin is an innovative third generation platinum compound with a powerful anti-neoplasm capability, a lack of cross drug resistance with cisplatin, a synergistic effect with fluorouracil (5-FU), and a satisfactory safety profile (7~9). This drug is an alkylating agent that inhibits DNA replication by forming adducts between adjacent guanine groups or between guanine and adenine. Moreover, many adducts of oxaliplatin appear to be more effective in inhibiting DNA synthesis than those of cisplatin inhibition (10~12). In addition, oxaliplatin has a more favorable toxicity profile than cisplatin (13).
The oxaliplatin/5-FU combination has proven to be an effective first- or second-line treatment for advanced colorectal cancer (14,15). Moreover, preliminary results from several recent studies suggest that various combinations of oxaliplatin and 5-FU may be equally effective in treating gastric cancer (16~19). This study was undertaken to evaluate the efficacy and toxicity of low dose leucovorin (LV) plus 5-FU in combination with oxaliplatin administered in fortnightly cycles (modified FOLFOX 4), as a salvage therapy for advanced gastric cancer patients.
MATERIALS AND METHODS
1) Eligibility criteria
The eligibility criteria are as follows: histologically confirmed adenocarcinoma of the stomach; previous treatment with other chemotherapy regimens; bidimensionally measurable lesions; no central nervous system metastases; no active infections; no serious or uncontrolled concurrent medical illnesses; no history of other malignancies; an Eastern Cooperative Oncology Group (ECOG) performance status of 0~2; and an age between 18 and 75 years. The institutional review board approved the protocol and written informed consent was obtained from each patient prior to study.
2) Treatment protocol and dose modification
On day 1, oxaliplatin (85 mg/m2) was administered via an intravenous (i.v.) infusion in 500 ml normal saline or dextrose over a 2-hour period. On days 1 and 2, LV (20 mg/m2) was administered as an iv bolus, which was immediately followed by 5-FU (400 mg/m2) that was given as a 10 minute iv bolus, and 5-FU (600 mg/m2) as a continuous 22 hour infusion with a light shield. Dose modifications to oxaliplatin or 5-FU were made for patients showing hematological, gastrointestinal, or neurological toxicity based on the most severe grade of toxicity that was encountered during the previous cycle. The patients were assessed before the beginning of each 2-week cycle using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC), with the exception in the case of neurotoxicity. In this case, the following oxaliplatin-specific scale was used: grade 1, paresthesias or dysesthesias of short duration, but resolving before the next cycle; grade 2, paresthesias persisting between cycles (2 weeks); and grade 3, paresthesias interfering with function. The treatment was delayed for up to 2 weeks for the following reasons: if the symptomatic toxicity persisted; if absolute number of neutrophils was <1,500/µl; or if the platelet count was <100,000/µl. The 5-FU dose was reduced by 25% for the subsequent courses if NCI-CTC grade 3 diarrhea, stomatitis, or dermatitis occurred. The dose of oxaliplatin was reduced by 25% in subsequent cycles if persistent paresthesias between the cycles or paresthesias with functional impairment lasting more than 7 days were encountered. The treatment was continued until there were signs of disease progression, the development of unacceptable toxic effects, or the patient refused further treatment.
3) Follow-up evaluation and assessment of response
Before each treatment course, a physical examination, routine hematology studies, chemistry, and chest X-ray were performed. The serum carcinoembryonic antigen (CEA) levels were determined after each cycle. CT scans were performed to determine the extent of the disease and the response after 4 cycles of chemotherapy, or sooner if there was evidence of any clinical deterioration.
The response was assessed using the WHO criteria (20). A complete response (CR) was defined as the disappearance of all evidence of the disease and the normalization of the tumor markers for a period of at least 4 weeks. A partial response (PR) was defined as a ≥ 50% decrease in the bidimensional tumor measurements, without the appearance of any new lesions or the progression of any existing lesion. Progressive disease (PD) was defined as any of the following: (1) a ≥ 25% increase in the sum of the products of all the measurable lesions, (2) the appearance of any new lesion, or (3) the reappearance of any lesion that had previously disappeared. Stable disease (SD) was defined as a tumor response that did not meet the criteria for a CR, PR, or PD.
The dose intensity (mg/m2/week) was calculated as the total cumulative dose divided by the treatment duration. The relative dose-intensity (RDI) was calculated by dividing the dose-intensity by the planned dose-intensity, and multiplying the result by 100. The planned dose-intensities, which is expressed as milligrams per square meter per week, were 1,000 for 5-FU and 42.5 for oxaliplatin.
4) Statistical methods
This trial was designed to detect a response rate of 25% compared with a minimal, clinically meaningful response rate of 5%. The two-stage optimal design proposed by Simon was used for this trial (21), with a statistical power of 90% for accepting the hypothesis and a 5% significance for rejecting the hypothesis. The total sample size required allowing for a follow-up loss rate of up to 10% was 33 patients with measurable disease. The variables included in this study were gender, age, previous adjuvant chemotherapy, and the baseline carcinoembryonic antigen (CEA) levels. The response rates with respect to the different variables were compared using Fisher's exact test. The time to progression and the overall survival were calculated using the Kaplan-Meier method. The time to progression was calculated from the date therapy had begun to the date of disease progression, and the overall survival was calculated from the date the therapy was begun to the date of death. All the data were analyzed using SPSS software (version 10.0, Chicago-IL)
RESULTS
1) Patient characteristics
Between December 2003 and December 2004, a total of 33 patients were treated at the department of Internal Medicine, Dong-A University Medical Center, Busan, Korea. Three patients were either to follow-up or had fewer than 2 cycles of chemotherapy. Therefore 30 patients were evaluated for their response and 33 patients were evaluated for their toxicity.
2) Objective tumor responses and survival
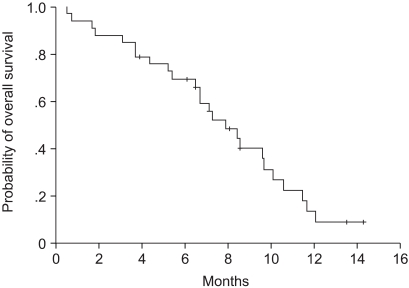
Of the 30 patients who could be evaluated for their tumor response, 8 achieved a partial response, with an overall response rate of 26.7% (95% confidence interval (CI): 20.5~32.7%), 15 (50%) showed stable disease, and 7 (23.3%) progressed during the course of treatment. The patients' age, gender, previous regimen, CEA level before chemotherapy, and the site of metastasis did not affect the response rate (Table 1). The median follow-up duration was 7.1 months, and the time to progression and overall survival were 3.5 months (95% CI: 2.6~4.4 months)(Fig. 1) and 7.9 months (95% CI: 5.9~9.9 months), respectively (Fig. 2). Docetaxel with cisplatin or irinotecan with 5-FU were administered to the 26 patients who progressed. Of these, only one patient achieved stable disease and others had progressive disease.
3) Toxicity
A total of 178 chemotherapy cycles were administered to the 33 patients. The median number of cycles was six ranging from 1 to 9. Table 2 summarizes the incidence of hematological toxicity and non-hematological toxicity. The major grade 3/4 hematological toxicity encountered included neutropenia (45.4%) and thrombocytopenia (3.0%). There were only 2 cycles of neutropenic fever. The most common non-hematological toxicity encountered was grade 1/2 nausea/vomiting (18.2%), diarrhea (12.1%), and neuropathy (15.2%). There was no treatment interruption or discontinuation for peripheral neuropathy. The relative dose intensities of oxaliplatin and 5-FU were 94% and 83% of the planned doses. There were no treatment related deaths. Most cycle delays (58 of 178) were due to treatment-related toxicity, and 10.6% (19 of 178) were for personal convenience. Of the 33 patients who received more than one cycle, 13 (39%) underwent at least one dose reduction among a total of 58 cycles (33%).
DISCUSSION
Despite the development of new palliative treatment modalities, the prognosis of advanced gastric cancer remains poor. Moreover, second-line chemotherapy in advanced gastric cancer patients who have progressed after the first-line chemotherapy remains a challenge (3). Clinical studies of second-line chemotherapy in gastric cancer patients have presented different results, partly due to the nature of the previous chemotherapy (4~6,16). Recently, second-line chemotherapeutic agents such as docetaxel (4) and irinotecan (5) were attempted in previously treated advanced gastric cancer patients, and their response rates as single agents have been reported to range from 5% to 20%. The response rate for docetaxel plus cisplatin as a second-line therapy in metastatic or recurrent advanced gastric cancer was reported to be 17.1% (6).
Oxaliplatin, 5-FU, and LV combination chemotherapy is accepted in colorectal cancer patients who are refractory to 5-FU and LV (15), and oxaliplatin in combination with 5-FU and LV (modified FOLFOX4) was previously examined as a first line treatment for advanced colorectal cancer (22). Recently, several studies using different administrative schedules of oxaliplatin and 5-FU reported its efficacy in treating gastric cancer (16~19), showing response rates of 43~44.9% (17~19), and 26% (16) as first- and second-line treatments, respectively.
This study treated advanced gastric cancer patients with this modified FOLFOX 4 regimen as a salvage treatment. The results suggest a response rate of 26.7% in this patient group, which is consistent with previous data (16). The median time to progression of the 33 patients in this study was 3.5 months, and the median overall survival was 7.9 months. The overall survival in this study was comparable to published second-line chemotherapy trial data (4,5,16). On the other hand, most patients (82%) had a good initial performance status and many (36%) had previously received third-line chemotherapy. The patients who received a curative resection did not respond to the treatment, which may be because these patients had already received adjuvant chemotherapy with 5-FU and cisplatin, and almost all (9/10) had previously received more than 2 regimens.
Owing to the patient selection protocols used in phase II studies, a comparison of the toxicity profiles in this study with those of other phase II studies that used different oxaliplatin and 5-FU administrative schedules is neither possible nor relevant. (16~19). Compared with a previous report (16), the fortnightly protocol in this study used lower levels of oxaliplatin (85 mg/m2 vs. 100 mg/m2), LV (30 mg/m2 vs. 150 mg/m2), and 5-FU (2.0 g/m2 vs. 2.4~3.0 g/m2). In this study, major grade 3/4 hematological toxicity including neutropenia (45.4%) and thrombocytopenia (3.0%) was encountered. This may be due to the bolus infusion of 5-FU used in our regimen and to the fact that patients had previously been heavily treated with other chemotherapy regimes. Nausea/vomiting and diarrhea were the most common non-hematological toxicities, and most were tolerable. In various FOLFOX trials, neurotoxicity is the most common side-effect leading to treatment discontinuation, but there was no grade 3/4 neurotoxicity encountered during the study. This may have been due to a relatively low cumulative dose of oxaliplatin (median 360 mg/m2) used in this series, because neuropathy is particularly prevalent for cumulative doses exceeding 540 mg/m2 (23). The treatment compliance for our regimen was good, and the median relative dose-intensity for oxaliplatin, and 5-FU were 94% and 83%, respectively.
As a result of the observed acceptable toxicity profiles, the modified FOLFOX4 represents an active and well-tolerated treatment regime as a salvage therapy in advanced gastric cancer patients. Nevertheless, new treatments and strategies will be needed to increase the survival and reduce the incidence of therapeutic toxicity.