AbstractPurposeThis study aimed to investigate the oncologic outcomes and prognostic factors of salvage treatments in patients with recurrent oropharyngeal squamous cell carcinoma (OPSCC) after radiotherapy (RT)-based treatment.
Materials and MethodsA cancer registry was used to retrieve the records of 337 patients treated with definitive RT or concurrent chemoradiotherapy (CRT) from 2008 to 2018 at a single institution. The poor-responder group (PRG) was defined as patients with residual or recurrent disease after primary treatment, and the oncologic outcomes for each salvage treatment method were analyzed. In addition, prognostic indicators of recurrence-free survival (RFS) and overall survival (OS) were identified in patients who underwent salvage treatment.
ResultsAfter initial (C)RT, the PRG comprised 71 of the 337 patients (21.1%): 18 patients had residual disease, and 53 had recurrence after primary treatment (mean time to recurrence 19.5 months). Of these, 63 patients received salvage treatment (surgery 57.2%, re-(C)RT 23.8%, and chemotherapy 19.0%), and the salvage success rate was 47.6% at the last follow-up. The overall 2-year OS for salvage treatments was 56.4% (60.8% for the salvage surgery group and 46.2% for the salvage re-(C)RT). Salvage surgery patients with negative resection margins had better oncologic outcomes than those with close/positive resection margins. Using multivariate analyses, locoregional recurrence and residual disease after primary surgery were associated with poor outcome after salvage treatment. In Kaplan-Meier analyses, p16 status was significantly associated with OS in the initial treatment setting but not in the salvage setting.
IntroductionThe incidence of oropharyngeal squamous cell carcinoma (OPSCC) has recently increased, largely due to the recent surge of human papillomavirus (HPV)–positive OPSCC [1,2]. Traditionally, the treatment of choice for OPSCC has been upfront surgery or radiation therapy (RT) with or without chemotherapy. However, successful application of organ preservation protocols in advanced laryngopharyngeal cancer treatment has led to increased utilization of upfront RT or chemoradiotherapy (CRT) over upfront surgery in OPSCC management [3,4]. In addition, HPV-positive OPSCC tends to show a more favorable response to upfront (C)RT than its HPV-negative counterpart. When upfront RT-based treatment is considered, some critical prognostic factors, such as age, initial tumor stage, and smoking history, have been identified [2,5].
The reported recurrence rates for OPSCC following upfront (C)RT range from 13% to 35%. The salvage option should be selected based on initial treatment modality, site of recurrence in relation to the previous RT target volume, and expected toxicity of the salvage modality [2,6–8]. For example, surgical salvage is the preferred modality for those with resectable locoregional recurrence following upfront RT-based treatment, given the increased risk of re-irradiation toxicity and the possibility of resistance to RT. As surgical oncologists are often confronted with OPSCC patients who need salvage surgery following upfront (C)RT, helpful decision-making criteria are needed. Many previous studies have suggested that salvage surgery could lead to fair oncologic outcomes for local and regional recurrence and distant metastasis [2,9–13]. Salvage (C)RT has been performed in select patients, but there is inadequate information regarding the oncologic outcomes in the re-RT setting. Furthermore, the clinical courses and risk factors of further recurrence and survival following salvage treatment have not been well established [10,11,13].
In the present study of OPSCC, we investigated (1) treatment outcomes and recurrence patterns following upfront RT-based treatment and (2) the types of salvage treatments provided, subsequent oncologic outcomes, and related prognostic factors.
Materials and Methods1. Study populationThe appropriate institutional review board approved this retrospective study (IRB No. 2021-12-151-001). Between January 2008 and December 2018, 337 primary OPSCC patients underwent upfront RT-based treatment at the authors’ institute. Following a thorough history and physical examination, all patients underwent histologic confirmation by biopsy and were evaluated by contrast-enhanced neck computed tomography (CT) and 5-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). In addition, patients with equivocal findings regarding neck lymph node metastasis in the initial imaging studies underwent ultrasonography-guided fine-needle aspiration cytology to confirm the presence or absence of lymphatic metastasis. The p16 tumor suppressor protein was used as a surrogate marker of the HPV status of the tumors: the presence of diffuse (at least 70%) and strong nuclear and cytoplasmic staining for p16 was considered positive. The p16 status of the tumors was classified as p16-positive, p16-negative, or unknown. We could not obtain the p16 status of some of the enrolled patients (unknown p16 status) because of lack of preoperative results and remaining paraffin-embedded tissue.
The enrolled patients’ level of functioning in terms of ability to care for themselves, daily activity, and physical ability was assessed at the times of primary and salvage treatment using the Eastern Cooperative Oncology Group (ECOG) performance scale (grade 0, 1 or 2). Following multidisciplinary tumor board discussion, upfront RT alone or CRT was administered to these patients. Patients with histological features other than those of squamous cell carcinoma, distant metastasis on the initial staging workup, recurrence following previous surgery as the initial treatment, and/or history of malignancy other than OPSCC were excluded from the current analyses.
Local recurrence refers to the return of cancer in the same area where it first started. Locoregional recurrence refers to the recurrence of cancer in the same area where it first started, as well as in nearby tissues or neck lymph nodes. Regional recurrence refers to the recurrence of cancer in neck lymph nodes, but not in the same location with primary tumor. Distant metastasis refers to the spread of cancer cells to distant parts of the body, such as the lungs, liver, or bones.
2. RT-based treatment schemeThere were progressive modifications of the RT techniques applied to patients throughout the study period. During the early study period, intensity-modulated RT was the preferred technique. However, after 2015, a combination of IMRT and intensity-modulated proton therapy was implemented when proton beam therapy equipment became available at our institute. In all patients, the RT plan was developed following a contrast-enhanced CT-based simulation with thermoplastic mask immobilization. Based on all clinical information, three levels of radiation target volumes were delineated according to our institutional selective neck irradiation policy: gross tumor volume (GTV), high-risk clinical target volume (HR-CTV), and low-risk clinical target volume (LR-CTV) [14]. The typical doses were designed to deliver 66–68.4 Gy to the GTV, 56–60 Gy to the HR-CTV, and 32–36 Gy to the LR-CTV, each delivered over 28–30 fractions. The same target delineation and dose prescription policies were applied regardless of the RT technique used and the HPV status. For the 295 patients (87.5%) who had cT3–4 and/or cN+ disease (as identified by the 7th edition American Joint Committee on Cancer staging system [15]), systemic chemotherapy was delivered concurrently with the RT course unless clinically contraindicated. There was no standard contraindication for chemotherapy during adjuvant radiation therapy. If deemed appropriate, chemotherapy may be omitted for patients with underlying renal disease, other comorbidities, or poor performance status after counseling with local medical experts, hemato-oncologists, and multidisciplinary tumor board meetings. Common chemotherapy regimens were two tri-weekly cycles of 100 mg/m2 cisplatin (212 patients, 71.8%), six weekly cycles of 59 mg/m2 cisplatin (59, 20.0%), and a 400 mg/m2 cetuximab loading dose followed by five weekly doses of 250 mg/m2 (24, 8.1%).
3. Salvage and palliative treatmentThe salvage and palliative treatment methods were determined after multi-disciplinary discussions involving head and neck surgeons, radiation oncologists, hemato-oncologists, pathologists, and radiologists. Treatment methods were determined by tumor location and extent of recurrence, previous radiation field, and surgical resectability based on radiologic examinations. Salvage surgery was performed when a recurrent tumor could be removed with a negative resection margin without significant morbidity, including major vessel damage. After a multi-disciplinary discussion, we also considered salvage surgery for cases with of oligometastasis.
The patients underwent re-radiation when surgery for the recurrent tumor was not feasible or the previous radiation field did not cover the recurrence sites. Palliative chemotherapy was performed when re-radiation or salvage surgery was not feasible, and distant metastasis was identified.
4. Follow-up protocolTwo consecutive post-RT response evaluations were performed for all patients: the first was neck contrast-enhanced CT imaging one month after RT completion, and the second was FDG-PET/CT imaging three months later. Response assignment in the current study was based on metabolic tumor volume (MTV) measured on FDG-PET/CT performed four months after upfront RT. A complete response (CR) was identified if the MTV was ≤ 40 mL, while residual disease (RD) was identified if the MTV was > 40 mL [16]. Regular follow-up assessments were scheduled at 3–6-month intervals, and all patients were divided into two groups: the good responder group (GRG) included the patients who initially achieved a CR and showed no recurrence through the last follow-up, while the poor responder group (PRG) included those who showed RD or developed subsequent recurrence after initially achieving a CR. When recurrence (and/or metastasis) was suspected, CT and PET-CT imaging was performed; in addition, tissue confirmation through biopsy or aspiration cytology was performed whenever feasible. Salvage therapy options for recurrent lesions were determined at the regular multidisciplinary conference after considering the recurrence nature and the patient’s general condition as mentioned above. We classified the salvage treatment outcomes based on survival status with no recurrence at more than 2 years after salvage therapy.
5. Statistical analysesWe compared the clinical factors between the GRG and PRG. Fisher’s exact test was used to compare the categorical variables, and the Mann-Whitney U test was used for continuous variables. Kaplan-Meier survival analyses with a log-rank test were performed to calculate the survival durations following initial and salvage treatments. The durations of recurrence-free survival (RFS) and overall survival (OS) after primary RT-based treatment were measured as the intervals between the dates of the final radiation treatment and of recurrence and death, respectively.
Univariate and multivariate Cox proportional hazards model analyses were performed to identify the clinical risk factors for RFS and OS. The hazard ratio (HR) and 95% confidence interval were estimated. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS ver. 25 (IBM Corp., Armonk, NY).
Results1. Outcomes of initial RT-based treatmentThe clinicopathological characteristics are summarized in Table 1. The mean ages of the GRG and PRG patients were 59.2 and 61.5 years, respectively, and there was a male preponderance in both groups. There were no differences in distribution of gender, smoking status, proportion of CRT, cT classification, and overall stages between the groups. Compared to the PRG, however, the GRG group had more patients with p16 positivity (58.3% vs. 38.0%, p=0.001) and cN1 category (26.7% vs. 8.5%, p=0.003). The mean follow-up period of all patients was 45.1±16.9 months: 47.7±15.5 months for the GRG and 35.7±18.9 months for the PRG.
The clinical outcomes following upfront RT-based treatment, with or without subsequent salvage treatment efforts, are illustrated in Fig. 1. Following upfront RT-based treatment, 319 patients (94.6%) achieved CR, 266 of whom (83.3%) had no evidence of disease through the final follow-up (GRG). The PRG consisted of 71 patients: 18 who did not achieve CR following upfront RT-based treatment (i.e., residual disease: 12 patients with partial responses and 6 with progressive disease) and 53 who developed recurrence at a mean of 19.5±20.7 months after achieving initial CR. The failure patterns after initial CR were local recurrence only in 13 patients, locoregional recurrence in 3, regional recurrence only in 13, and distant metastasis in 24.
2. Oncologic outcomes following salvage treatmentIn the PRG, eight patients refused further salvage management, and the remaining 63 underwent salvage treatment (Tables 2 and 3, Fig. 1).
The patients with recurrent oropharyngeal cancer (n=71) were classified as having undergone salvage surgery (n=36), salvage (chemo)radiation (n=15), chemotherapy (n=12), or no further treatment (n=8). The age at recurrence was similar in the patients who underwent salvage surgery (55.9±12.3 years), salvage (chemo)radiation (63.0±14.3 years), or chemotherapy (56.6±12.6 years). However, the patients without further treatment (72.5±9.4) were significantly older (p=0.042) than the others. The ECOG performance status was also similar between the patients who underwent salvage surgery, (chemo)radiation, or chemotherapy, but patients receiving no further treatment had significantly poorer performance status (p=0.027).
The salvage therapy modalities for 13 patients with local recurrence were surgery in 12 and re-RT in one, and the OS 2 years after salvage treatment was 76.9%. Among three patients with locoregional recurrence, all underwent salvage surgery, and two showed no further recurrence through the final follow-up, while the other developed regional recurrence after salvage treatment (2-year OS of 66.7%). The salvage therapy types for 12 patients with regional recurrence were surgery in five, re-(C)RT in seven, and none in one (2-year OS of 79.4%). The salvage therapy types for 24 patients with distant metastases were surgery in three, re-RT in six, chemotherapy in 10, and none in five (2-year OS of 42.1%).
Successful salvage (alive without recurrence more than 2 years after salvage treatment) was achieved in 30 of 63 patients (47.6%) following salvage treatment; however, recurrence after salvage treatment occurred in 33 patients, due to residual disease in 10, local failure in nine; locoregional failure in six; regional failure in five; and distant metastasis in three. Regarding the salvage options, OS 2 years after surgery or re-(C)RT salvage treatment was 60.8% and 46.2%, respectively.
3. Oncologic outcomes and resection margin of salvage surgerySalvage surgery for recurrence or residual disease after primary treatment was performed in 36 patients, and 18 (50.0%) were alive without recurrence more than 2 years after salvage surgery (Fig. 2). Among 12 patients who underwent surgery for local recurrence, seven (61.5%) were successfully salvaged (alive without recurrence more than 2 years after salvage treatment), while five (38.5%) experienced recurrence after salvage treatment. There were three cases of local recurrence after salvage treatment, all of whom had adverse pathologic features of positive or close resection margins in the surgical specimen. Another patient with close resection margins at salvage surgery experienced locoregional recurrence in the contralateral neck site.
Salvage surgery was successful in two of three patients with local-regional recurrence, a success rate of 66.7%. One patient who failed after salvage surgery experienced a second locoregional recurrence. Among five patients with regional recurrence, salvage surgery was successful in two (40.0%). Among the patients who developed treatment failure following salvage surgery, the proximity of the recurrent tumor to the carotid hindered successful removal during the first salvage surgery, resulting in inadequate resection margins. Among 11 patients with residual disease, eight (61.5%) failed following salvage surgery, seven of which had positive or close resection margins and developed local and loco-regional recurrence, while one with no post-surgical adverse features nonetheless developed distant metastasis.
Of note, salvage surgery was performed in three patients with distant metastases, among whom two with oligo-metastases in the lung showed no recurrence more than 2 years after metastasectomy.
Patients with negative resection margins (n=21, 58.3%) after salvage surgery had better oncologic outcomes (p=0.007) than patients with positive/close resection margins (n=15, 41.7%). In addition, regional recurrence and residual disease occurred frequently in patients with close/positive resection margins due to their proximity to vital structures including major vessels (Table 4).
4. Prognosticators of RFS and OS after salvage treatmentCox proportional hazards model analyses were used to identify probable prognostic factors for RFS and OS (Table 5) after salvage treatment. With respect to RFS, age, sex, smoking, type of salvage treatment, and p16 status did not show any significance in univariate and multivariate analyses. Recurrence site was associated with worse RFS, where locoregional recurrence (HR, 6.296; p=0.040) and residual disease (HR, 5.584; p=0.004) were more likely factors than local recurrence. With respect to the type of salvage, patients who underwent chemotherapy (HR, 2.849; p=0.025) had worse OS than those who underwent salvage surgery.
Overall survival following the initial and salvage treatments as estimated using Kaplan-Meier analyses is illustrated in Fig. 3. In the initial treatment setting, patients with positive or unknown p16 status had better OS than p16-negative patients (p=0.001). There was no significant difference, however, with respect to initial tumor stage (p=0.154). In contract, p16 status and recurrence site were not significantly associated with OS in the salvage setting.
DiscussionThis study aimed to assess the outcomes following salvage treatment of OPSCC and the to identify prognostic factors for successful salvage of recurrent OPSCC following upfront RT-based treatment. In this retrospective analysis, the PRG (either residual disease or subsequent recurrence after initial CR) comprised 71 of 337 patients (21.1%). The most common location of recurrence was distant metastasis, followed by local and regional sites. Overall success of salvage (alive without recurrence more than 2 years after salvage treatment) in our cohort was 47.6%, and the 2-year OS and RFS of patients who underwent salvage treatment were 56.4% and 42.0%, respectively. Salvage surgery was the preferred treatment modality, with a 2-year OS of 60.8%. Locoregional recurrence and residual disease were associated with poorer RFS than at other sites, and the p16-negative patients had worse OS than p16-positive patients in the initial treatment setting, although this result was not reproduced in the salvage setting.
For most advanced head and neck cancer patients, RT is the preferred treatment because of the presumed organ preservation potential and more favorable functional outcomes than for upfront radical surgery. However, nearly 40% of patients with stage III–IV disease relapse following upfront RT, resulting in the demand for salvage treatment. The treatment strategy selected for recurrent OPSCC is prognostically significant. Based on previous reports, salvage surgery for recurrent OPSCC has provided significantly more favorable oncologic outcomes than other options, consistent with the findings of the present study (2-year OS of 60.8% and 46.2% following salvage surgery and re-(C)RT, respectively) [17–19]. In addition, 2-year OS following salvage surgery in the present study was comparable with previous systematic review data (2- and 5-year OS of 52%, and 30%, respectively) [20].
In our study, 36 patients underwent salvage surgery for local, locoregional, regional recurrence, or distant metastasis. After salvage surgery, 18 patients (50.0%) were alive without recurrence more than 2 years after salvage treatment, and the other 18 (50.0%) had developed recurrence after salvage treatment. A major cause of failure was inadequate resection margin during salvage surgery. The reasons for insufficient tumor resection were (1) poor surgical exposure to the recurrent tumor, (2) difficulty in accurately defining the tumor boundary (clinically and pathologically), and (3) proximity of the tumor to vital structures, such as carotid vessels. Similarly, a previous analysis of 434 recurrent OPSCC patients reported that 66.7% of the patients experienced recurrence following salvage surgery, and surgical margin status was one of the significant risk factors for recurrence and death [21]. Another study reported a positive surgical margin as the only predictor of reduced disease-free survival, and a positive margin was associated with an 8.4-fold greater risk of recurrence than a negative margin [22]. In addition, frozen-section margin evaluation during salvage surgery is challenging, especially within the previously irradiated operative field, making it more difficult to accurately delineate the tumor boundary.
Furthermore, salvage surgery for recurrent OPSCC is frequently associated with significant morbidity and mortality [21,23–25]. It has been reported that 22% of patients require tube feeding before salvage surgery, increasing to 64% following salvage surgery [21]. Likewise, other reports demonstrated that surgery-based treatment was associated with greater swallowing-related morbidity than RT-based therapy in both the initial and salvage treatment settings [24,25]. Therefore, candidates for salvage surgery must be selected carefully, considering the surgical accessibility and resectability of recurrent tumors and subsequent expected morbidities.
Salvage surgery could be an effective option for recurrent OPSCC, even in distant metastasis [10,18,19]. A study of salvage outcomes in HPV-positive OPSCC demonstrated that nine of 23 patients with distant oligo-metastatic disease achieved successful surgical salvage, and 39.1% of patients achieved disease control through the final follow-up [10]. In our study, three patients with distant metastasis underwent salvage surgery, among whom two achieved successful salvage by removing single lung metastasis. However, a more efficient strategy for managing distant recurrence is necessary as this is a common problem in OPSCC patients regardless of HPV status [9].
In our cohort, 15 patients, most with regional recurrence and distant metastasis, underwent re-RT or CRT as the salvage option. Although re-RT may be associated with increased morbidity, including radiation-induced necrosis in the previously irradiated regions, it may provide acceptable oncologic outcomes, given the 2-year OS of 46.2% for salvage treatment in this study. Therefore, re-(C)RT may be an excellent salvage option in patients who respond poorly to initial treatment, especially when adequate surgical exposure, resection, and surgical margins are not likely.
Prognostic factors in the salvage treatment setting have been less frequently investigated than those in the initial treatment setting, and some uncertainty remains. A retrospective study of 86 recurrent OPSCC patients reported that HPV-positive tumor status and surgical recurrence reduced OS [9]. Another multicenter retrospective study of 108 recurrent OPSCC patients demonstrated that longer time to recurrence, primary surgical treatment, and surgical salvage were associated with improved OS [18]. An analysis of 69 patients who underwent salvage surgery for recurrent OPSCC reported that HPV status and smoking status were not significant, and only tumor stage and gastrostomy dependency significantly impacted OS [13]. One previous study of surgical salvage for recurrent OPSCC reported that cervical lymph node metastasis and clinical stage were substantial risk factors of reduced OS [13]. Another study of recurrent HPV-positive OPSCC showed that solitary recurrence was associated with more favorable than multi-site recurrence [10]. In the present study, neither p16 status nor site of recurrence had any prognostic significance for OS in the salvage treatment setting. Similar to the findings of our study, a multicenter retrospective study for recurrent OPSCC showed that salvage treatment outcomes did not differ based on p16 status [9].
A major drawback of the present study is that it was a retrospective analysis of the cancer registry from a single institution. Therefore, the outcomes of this study must be interpreted cautiously in light of the risk of selection bias.
In conclusion, successful salvage was achieved in 56.4% patients who underwent salvage surgery and radiation treatment for recurrent OPSCC after primary radiation treatment. Salvage treatment methods should be carefully selected as recurrence site was a significant prognostic indicator of RFS. The findings of our study indicate that salvage surgery should be considered when a clear resection margin can be safely achieved. Wide exposure to the surgical field and accurate delineation of the tumor boundaries were critical in reducing subsequent failure. In addition, salvage radiation treatment was successful in selected cases. Unlike the initial treatment setting, p16 status had no prognostic significance in the salvage setting, and the site of recurrent tumors was a critical factor for RFS.
NotesEthical Statement This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center in Korea (IRB no. 2021-12-151-001). The need for informed consent from the study participants was waived as this study used deidentified administrative data. Author Contributions Conceived and designed the analysis: Choi N, Kim HJ, Yi H, Kim H, Kim TH, Jeong HS, Son YI, Baek CH, Oh D, Ahn YC, Chung MK. Collected the data: Choi N, Kim HJ, Yi H, Kim H, Kim TH, Jeong HS, Son YI, Baek CH, Oh D, Ahn YC, Chung MK. Contributed data or analysis tools: Choi N, Kim HJ, Ahn YC, Chung MK. Performed the analyses: Choi N, Kim HJ, Yi H, Kim H, Kim TH, Jeong HS, Son YI, Baek CH, Oh D, Ahn YC, Chung MK. Wrote the paper: Choi N, Kim HJ, Ahn YC, Chung MK. Fig. 1Clinical outcomes and types of salvage treatment of patients with initial radiation-based treatment for oropharyngeal cancer (n=337). CR, complete remission; (C)RT, (chemo)radiation therapy; CT, chemotherapy; NED, no evidence of recurrent disease; RD, residual disease. 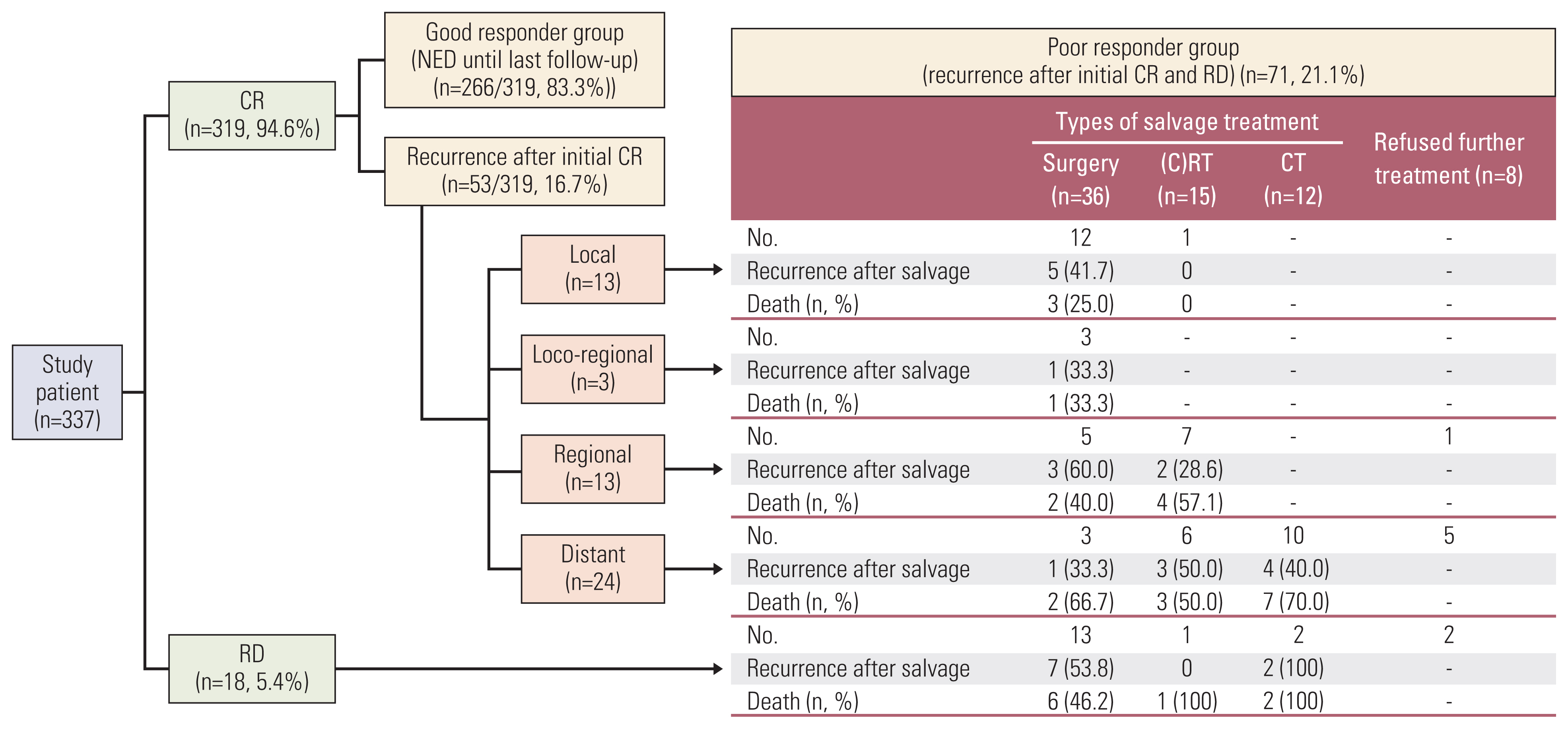
Fig. 2Outcomes of salvage surgery for local, regional, and locoregional recurrence and distant metastasis (n=36). 
Fig. 3Kaplan-Meier survival analyses according to initial tumor stage, p16 status, and site of recurrence among patients in the initial treatment setting (n=337) (A, B) and salvage treatment setting (n=63) (C, D). 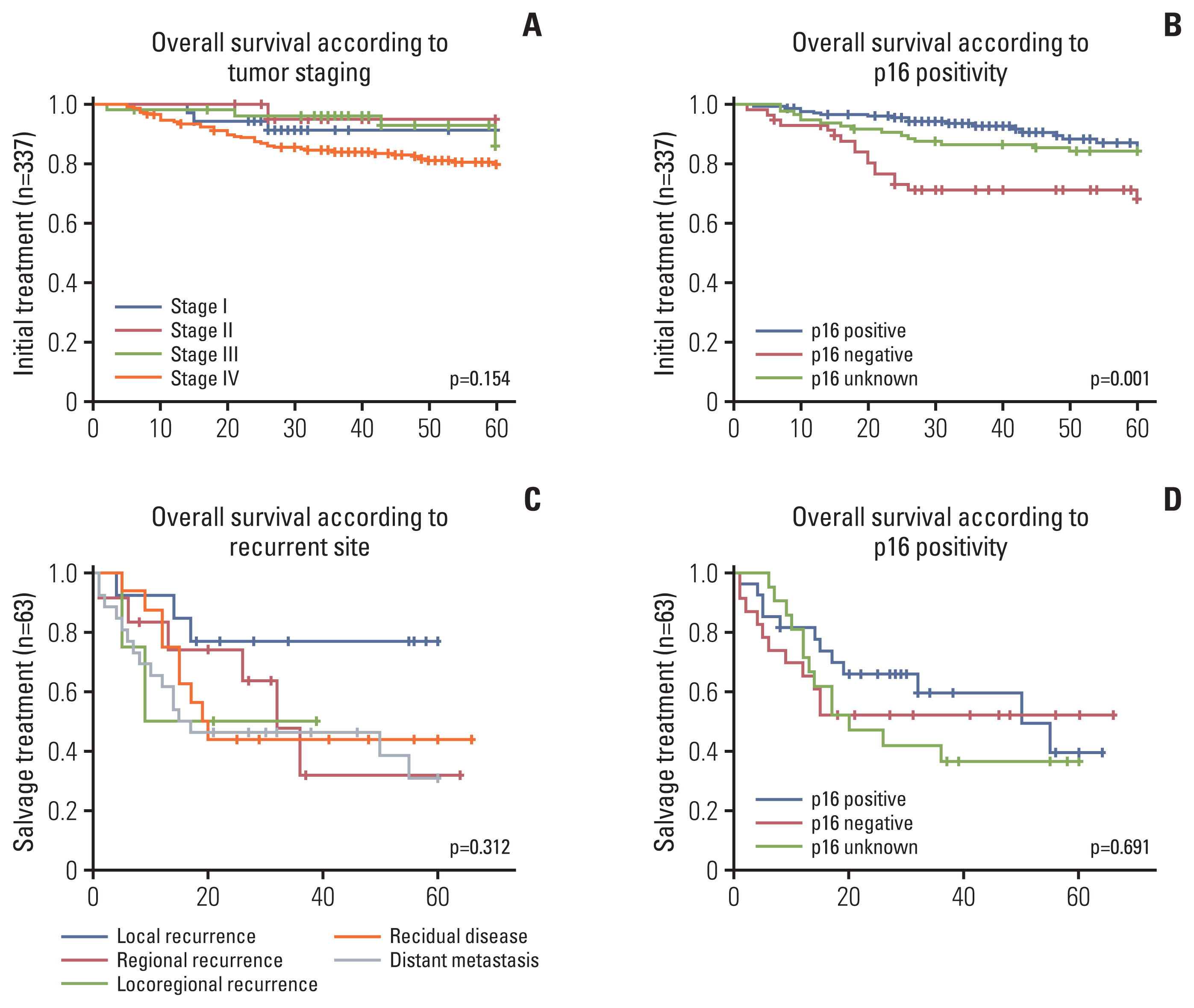
Table 1Clinical characteristics of study patients with oropharyngeal cancer initially treated with radiotherapy (n=337)
Table 2Clinical characteristics at the time of recurrence of oropharyngeal cancer (n=71) ECOG performance status scale: 0 (fully active), 1 (restricted with respect to physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature), 2 (ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours). ECOG, Eastern Cooperative Oncology Group; SD, standard deviation. Table 3Outcomes of salvage and palliative treatments of the poor-responder group (n=63) Table 4Oncologic outcomes and resection margins in patients who underwent salvage surgery (n=36)
Table 5Univariate and multivariate analyses using a Cox proportional hazards model to assess recurrence-free survival and overall survival in patients who underwent salvage treatment for recurrent oropharyngeal squamous cell carcinoma (n=63) References1. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301.
2. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
3. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985–2001. Laryngoscope. 2007;117:16–21.
4. Cohan DM, Popat S, Kaplan SE, Rigual N, Loree T, Hicks WL Jr. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg. 2009;17:88–94.
5. Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9.
6. Roosli C, Studer G, Stoeckli SJ. Salvage treatment for recurrent oropharyngeal squamous cell carcinoma. Head Neck. 2010;32:989–96.
7. Kano S, Homma A, Hayashi R, Kawabata K, Yoshino K, Iwae S, et al. Salvage surgery for recurrent oropharyngeal cancer after chemoradiotherapy. Int J Clin Oncol. 2013;18:817–23.
8. Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50.
9. Culie D, Lisan Q, Leroy C, Modesto A, Schiappa R, Chamorey E, et al. Oropharyngeal cancer: first relapse description and prognostic factor of salvage treatment according to p16 status, a GETTEC multicentric study. Eur J Cancer. 2021;143:168–77.
10. Christopherson KM, Moreno AC, Elgohari B, Gross N, Ferrarotto R, Mohamed ASR, et al. Outcomes after salvage for HPV-positive recurrent oropharyngeal cancer treated with primary radiation. Oral Oncol. 2021;113:105125.
11. Heft Neal ME, Brennan J, Brenner JC, Shuman AG, Chinn SB, Stucken CL, et al. Predictors and prevalence of nodal disease in salvage oropharyngectomy. Ann Surg Oncol. 2020;27:451–7.
12. Hay A, Simo R, Hall G, Tharavai S, Oakley R, Fry A, et al. Outcomes of salvage surgery for the oropharynx and larynx: a contemporary experience in a UK Cancer Centre. Eur Arch Otorhinolaryngol. 2019;276:1153–9.
13. Sweeny L, Rosenthal EL, Clemons L, Stevens TM, Cook McIntosh ER, Carroll WR. Outcomes after surgical salvage for recurrent oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;60:118–24.
14. Yoon HG, Ahn YC, Oh D, Noh JM, Park SG, Nam H, et al. Early clinical outcomes of intensity modulated radiation therapy/intensity modulated proton therapy combination in comparison with intensity modulated radiation therapy alone in oropharynx cancer patients. Cancers (Basel). 2021;13:1549.
15. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
16. Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–8.
17. Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–73.
18. Guo T, Qualliotine JR, Ha PK, Califano JA, Kim Y, Saunders JR, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121:1977–84.
19. Patel SN, Cohen MA, Givi B, Dixon BJ, Gilbert RW, Gullane PJ, et al. Salvage surgery for locally recurrent oropharyngeal cancer. Head Neck. 2016;38(Suppl 1):E658–64.
20. Kao SS, Ooi EH. Survival outcomes following salvage surgery for oropharyngeal squamous cell carcinoma: systematic review. J Laryngol Otol. 2018;132:299–313.
21. Zafereo ME, Hanasono MM, Rosenthal DI, Sturgis EM, Lewin JS, Roberts DB, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115:5723–33.
22. Joseph AW, Guo T, Hur K, Xie Y, Yin L, Califano JA, et al. Disease-free survival after salvage therapy for recurrent oropharyngeal squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1501–9.
23. Kostrzewa JP, Lancaster WP, Iseli TA, Desmond RA, Carroll WR, Rosenthal EL. Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal cancer. Laryngoscope. 2010;120:267–72.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||