AbstractPurposeFrequent neutropenia hinders uninterrupted palbociclib treatment in patients with hormone receptor (HR)–positive breast cancer. We compared the efficacy outcomes in multicenter cohorts of patients with metastatic breast cancer (mBC) receiving palbociclib following conventional dose modification or limited modified schemes for afebrile grade 3 neutropenia.
Materials and MethodsPatients with HR-positive, human epidermal growth factor receptor 2–negative mBC (n=434) receiving palbociclib with letrozole as first-line therapy were analyzed and classified based on neutropenia grade and afebrile grade 3 neutropenia management as follows: group 1 (maintained palbociclib dose, limited scheme), group 2 (dose delay or reduction, conventional scheme), group 3 (no afebrile grade 3 neutropenia event), and group 4 (grade 4 neutropenia event). The primary and secondary endpoints were progression-free survival (PFS) between groups 1 and 2 and PFS, overall survival, and safety profiles among all groups.
ResultsDuring follow-up (median 23.7 months), group 1 (2-year PFS, 67.9%) showed significantly longer PFS than did group 2 (2-year PFS, 55.3%; p=0.036), maintained across all subgroups, and upon adjustment of the factors. Febrile neutropenia occurred in one and two patients of group 1 and group 2, respectively, without mortality.
IntroductionHormone receptor (HR)–positive/human epidermal growth factor receptor 2 (HER 2)–negative breast cancer constitutes 60%–70% of all metastatic breast cancers (mBCs), and endocrine therapy plus cyclin-dependent kinase 4/6 (CDK4/6) inhibitor treatment is now recognized as the gold standard for first-line treatment of HR-positive breast cancer (BC) without visceral crisis. Landmark clinical trials have shown improvements in progression-free survival (PFS) and overall survival (OS) following CDK4/6 inhibitor treatment in mBCs [1–7]. More recently, two phase III adjuvant CDK4/6 inhibitor clinical trials reported discordant results: no invasive disease-free survival (iDFS) gain by adjuvant palbociclib (PALLAS), in contrast to early signals of iDFS improvement by abemaciclib (MonarchE) [8,9]. The lack of benefit from palbociclib treatment in the PALLAS trial has not been fully explained; however, the early discontinuation rate due to adverse events (AEs; 45.1%) and cumulative proportion of dose reduction (100 mg in 55.4% and 75 mg in 34.3%) of palbociclib within 2 years emphasize that finer palbociclib dose adjustment may be required for potential survival improvement [10].
Palbociclib suppresses BC cell growth and endocrine resistance by inhibiting the CDK4/6-retinoblastoma–E2 factor axis [11]. However, palbociclib inevitably suppresses CDK4/6-dependent hematopoietic cell proliferation, resulting in frequent grade ≥ 3 neutropenia events. Despite the high incidence of this adverse effect of palbociclib treatment, patients are rarely febrile (1%–2%); this does not commonly lead to mortality, as the molecular mechanism of palbociclib-induced neutropenia involves the cytostatic arrest of hematopoietic cells, rather than cytotoxic cell death caused by chemotherapy [12–14]. Based on the mechanistic differences in bone marrow toxicity between CDK4/6 inhibitors and chemotherapy, as well as the rare incidence of palbociclib-induced febrile neutropenia, we suggest that the dose reduction scheme for palbociclib could be more permissive to neutropenia than that indicated by existing guidelines.
During drug labeling of palbociclib, researchers recommend repeated complete blood cell count monitoring after 1 week for patients who experience grade 3 neutropenia, followed by withholding palbociclib until at least grade 2 recovery. Researchers also recommend that dose reduction be considered in cases of prolonged (> 1 week) or recurrent grade 3 neutropenia [15].
In our previous study (Ham et al. [14]), we proposed a new palbociclib dose modification scheme that avoided dose delays or reductions in afebrile grade 3 neutropenia by analyzing 107 palbociclib-treated patients with BC. Although we strictly omitted dose interruption or reduction in afebrile grade 3 neutropenia, the patients remained safe and stable for a prolonged period of palbociclib treatment without neutropenia-related infection events, hospitalization, or death. Additionally, we anticipated that the increased dose intensity based on the new scheme could lead to enhanced efficacy of palbociclib, although this had not been explored because of the limited sample size and follow-up duration in the previous study.
Therefore, we performed a retrospective multicenter cohort study to evaluate the difference in the PFSs between real-world patients with HR-positive, HER2-negative mBC who received palbociclib following the conventional dose modification scheme (delay or reduction in afebrile grade 3 neutropenia) and those who received palbociclib with the limited dose modification scheme (no delay or reduction in afebrile grade 3 neutropenia). To the best of our knowledge, this is the first study to analyze the dose-dependent efficacy of palbociclib for afebrile grade 3 neutropenia.
Materials and Methods1. PatientsA cohort of consecutive patients with mBC who received palbociclib combined with letrozole at four major cancer centers (Yonsei Cancer Center, Asan Medical Center, Gangnam Severance Hospital, and Seoul St. Mary’s Hospital) in the Republic of Korea was retrospectively analyzed. Only patients who received letrozole and palbociclib in a standard clinical practice, instead of clinical trials, were included. The Institutional Review Board of each institution approved the study protocol. The requirement for informed consent was waived because this study was a retrospective review of medical records. Patients with a confirmed diagnosis of estrogen receptor–positive and/or progesterone receptor– positive and HER2-negative BC were included. Those who received palbociclib and letrozole as first-line therapy for mBC with adequate baseline hematologic function were analyzed: (1) absolute neutrophil count (ANC) ≥ 1,500 cells/mm3, (2) platelets ≥ 100,000 cells/mm3, and (3) hemoglobin ≥ 9 g/dL.
2. Patient grouping and endpoint evaluationsPatients received 125 mg of palbociclib daily for 3 weeks and then rested for 1 week (4-week cycle); they also received 2.5 mg of letrozole per day (continuous treatment). All premenopausal patients underwent bilateral salpingo-oophorectomy before treatment. Only surgical ovarian suppression, not chemical ovarian suppression by a gonadotropin-releasing hormone agonist, was allowed before palbociclib use in premenopausal patients by the National Health Insurance of the Republic of Korea. Laboratory tests were performed according to the institutional protocol and at the clinician’s discretion. To evaluate the survival impact of the palbociclib dose reduction scheme, we categorized the patients into four groups according to neutropenia grade and whether the palbociclib dose was modified for afebrile grade 3 neutropenia within the first five cycles: group 1 (patients who received a maintained palbociclib dose with afebrile grade 3 neutropenia, representing the limited dose modification scheme) (Table 1), group 2 (patients who experienced any dose modification with afebrile grade 3 neutropenia, representing the conventional dose modification scheme), group 3 (patients without any event of afebrile grade 3 or 4 neutropenia), and group 4 (patients who presented with grade 4 neutropenia [ANC ≤ 500 cells/mm3] without any grade 3 neutropenia event [500 cells/mm3 < ANC ≤ 1,000 cells/mm3]). To avoid selection bias, we compared patient survival according to dose modification status within the first five cycles rather than the whole period, as suggested by Berek et al. [16], who found that frequent dose modification led to a prolonged survival follow-up period. We set the fifth cycle as the cutoff because 45.0% (100 mg in 37.4%, 75 mg in 7.6%) of the dose reductions occurred within the first five cycles, after which the dose was maintained for the majority of patients (65.9%).
The primary endpoint was the PFS difference between group 1 (limited dose modification scheme) and group 2 (conventional dose modification scheme), and the secondary endpoints included PFS and OS differences in all groups and the safety profiles of each group. Response evaluation was performed based on computed tomography, magnetic resonance imaging, and/or whole-body bone scans every two-four treatment cycles per the institutional protocol. Toxicity was assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 5.0. The treatment was administered until progressive disease, or unacceptable toxicity was observed.
The study protocols adhered to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
3. Statistical analysisPFS and OS were defined as the duration from the first palbociclib administration to disease progression or death and until death from any cause, respectively. Patients who did not experience disease progression or death were censored on the date of the last visit. Baseline characteristics and toxicities are presented as counts with percentages and compared using the chi-square test or Fisher’s exact test. The Kaplan-Meier method was used to estimate survival, and the log-rank test was applied to determine the difference in survival between the groups. The Cox proportional hazards model was used in the univariate and multivariate analyses to assess the significant prognostic factors associated with PFS and OS. Two-sided p-values of < 0.05 were considered statistically significant. All statistical analyses and graphing were performed using IBM SPSS ver. 25 (IBM Corp., Armonk, NY) and the R software (ver. 4.1.0, R Foundation for Statistical Computing, Vienna, Austria).
Results1. Patients and baseline characteristicsData from 499 patients with HR-positive/HER2-negative mBC treated at four institutions from January 2017 to September 2020 were collected for the study, and 434 patients were eligible for the analysis. Sixty-five patients who met the following criteria were excluded: patients who were not followed up for > 3 months due to either patient withdrawal or discontinuation of clinical visits (n=18); who experienced progression < 1 month of palbociclib treatment (dose reduction analysis was not applicable) (n=6); with incomplete palbociclib dosing data (e.g., transfer from other hospitals in the middle of palbociclib treatment) (n=6); whose palbociclib dose was not reduced sequentially (two levels of dose reduction at once or starting dose other than 125 mg/day) (n=32); and who did not undergo dose reduction for febrile neutropenia (n=3).
Patients were categorized into four groups according to neutropenia events within the first five cycles, and the palbociclib dose modification scheme was implemented as described before; 174 (40.1%) patients were in group 1, 128 (29.5%) in group 2, 102 (23.5%) in group 3, and 30 (6.9%) in group 4 (Fig. 1). The median age was 54 years (range, 28 to 87 years), and all the patients were women. There were 252 patients (58.1%) with visceral metastasis and 100 patients (23%) with bone-only disease (Table 2). Group 1 patients showed a lower proportion of bone-only disease than group 2 patients (18.4% vs. 31.2%, p=0.009). The baseline clinical characteristics between groups 1 and 2 were not significantly different (S1 Table).
2. Palbociclib dose profilePalbociclib dose reduction for any reason was applied to 272 patients (62.7%) during the entire treatment period, and dose delay was applied to 181 patients (42.2%) in all the groups (Table 3). The median time to the first dose reduction for all eligible patients was 3 months (2–5 months), and the median time to the second dose reduction was 9 months (range, 2 to 30 months). The cumulative incidence of palbociclib dose reduction in group 1 was significantly lower than that in group 2 (Fig. 2).
The mean and median dose intensity was different depending on the group, and the difference was statistically significant (p < 0.001). Group 1 had higher mean and median dose intensities than did group 2, and this difference was statistically significant; mean dose intensity is 94.82% vs. 77.25%, and median dose intensity 100% (62.57–100) vs. 80.69% (61.35–94.16), that is group 1 vs. group 2, respectively (p < 0.001) (Tables 4 and 5).
S2 Fig. shows the proportion of palbociclib doses (125, 100, and 75 mg) in each cycle. Dose reductions tended to occur early, with 45.0% of the events occurring in the first five cycles (100 mg in 37.4% and 75 mg in 7.6%). During the 12th cycle of treatment, 70.5% (105/149 at-risk patients) of group 1 patients remained on 125 mg of palbociclib, whereas no patient remained on the 125 mg dose, and 66.3% of the patients (65/98 at-risk patients) were administered 100 mg of palbociclib in group 2.
3. Survival analysisAt the data collection cutoff in November 2020, 146 events comprising disease progression or death occurred in 434 patients, and 288 patients (66.4%) were still receiving palbociclib with letrozole. The median follow-up duration for all patients was 23.7 months (95% confidence interval [CI], 21.6 to 24.9), and the median PFS was 37.8 months. We compared patients in groups 1 and 2 to evaluate the survival impact of dose modification on grade 3 neutropenia within the first five cycles. The median follow-up durations of groups 1 and 2 were 24.1 months (range, 21.6 to 25.8) and 24.4 months (range, 22.9 to 26.6), respectively. Group 1 patients showed significantly longer PFS than group 2 patients (p=0.036, log-rank), and the 2-year PFS rate was 67.9% in group 1 and 55.3% in group 2. Difference in OS between groups 1 and 2 (p=0.253, 2-year OS rate: 95.4% in group 1 and 90.1% in group 2) (Fig. 3A and B) was not significant. A greater increase in the PFS trend of group 1 patients than of group 2 patients was observed across all subgroups (Fig. 4). The PFS improvement was most pronounced in de novo stage IV patients (PFS: hazard ratio, 0.354; 95% CI, 0.167 to 0.750; OS: hazard ratio, 0.183; 95% CI, 0.037 to 0.909) and was also observed in the young-age, non-bone-only disease, and multiple organ metastases subgroups, which were previously defined as high-risk groups. Cox regression analysis was performed to determine the PFS difference between groups 1 and 2 patients (Table 6), and group 1 patients showed significantly longer PFS rates than group 2 patients, independent of adjusting factors such as age, disease status, visceral metastasis, and the number of organ involvements (hazard ratio, 0.665; 95% CI, 0.447 to 0.989; p=0.044). The overall survival trend by subgroup of group 1 and 2 patients is shown in S3 Fig.
4. AEs: hematologic and non-hematologic toxicities and hospitalizationsThe incidence of all-grade AEs in group 1 patients was not different from that in group 2 patients (Table 7). The incidences of grade ≥ 3 anemia and thrombocytopenia were similar between groups 1 and 2, without any hospitalization events for anemia or thrombocytopenia. There were four cases (0.9%) of febrile neutropenia among all patients, one in group 1, two in group 2, and one in group 4, which resolved without mortality or long-term morbidity. Palbociclib was permanently discontinued because of AEs in 2.8% of cases. Common non-hematologic AEs were fatigue, oral mucositis, alopecia, and nausea. The following five hospitalization events occurred in non-hematologic AEs in groups 1 and 2 (Table 3): one patient each for pneumonitis, aspartate aminotransferase/alanine aminotransferase elevation, and urinary tract infection in group 1 and one patient each with gastric ulcer bleeding and anorexia in group 2. The platelet count of the patient with bleeding from the gastric ulcer was within the normal range. Most AEs were manageable by reducing or delaying the palbociclib dose.
DiscussionEndocrine therapy combined with CDK4/6 inhibitors is the standard first-line treatment for HR+ HER2− mBC. However, neutropenia frequently interrupts the planned palbociclib dosing in patients with mBC. In this study, we hypothesized that omitting palbociclib dose reduction or delay in afebrile grade 3 neutropenia may lead to increased efficacy of palbociclib in patients with mBC. We found a significantly longer PFS in the patient groups managed with the limited dose modification scheme of palbociclib upon afebrile grade 3 neutropenia than in the conventional dose modification group. This PFS improvement was observed independently of baseline clinical factors, including age, disease status, visceral metastasis, and the number of organs involved. Although the palbociclib dose was maintained in grade 3 neutropenia, the limited-dose scheme did not adversely affect the hematologic and non-hematologic safety profiles, and febrile neutropenia rarely developed in these patients. The 2-year PFS rate in the limited modification group was approximately 12% higher (67.9% in group 1 vs. 55.3% in group 2) than that in the conventional dose modification group. Combined with our previous proposal of a new dose modification scheme (Ham et al. [14]), this study highlights the necessity of palbociclib dose maintenance in afebrile grade 3 neutropenia to enhance the convenience and dose intensity of palbociclib therapy in patients with mBC.
The primary goal of this study was to compare the effects of maintained versus modified palbociclib treatment in patients with afebrile grade 3 neutropenia (groups 1 and 2). Comparison between groups 3 and 4 was not a primary hypothesis, but it was included to provide complete information of all the collected data regarding neutropenia status and its prognosis following palbociclib treatment (S4 Fig.). The results of groups 3 and 4 support those of groups 1 and 2; the patient prognosis was dependent on the degree of palbociclib exposure. The presentation of grade 4 neutropenia indicates that these patients were exposed to higher blood concentrations of palbociclib than patients who did not present with grade 3 neutropenia [17]. Group 1 patients would have maintained a higher concentration of palbociclib by maintaining a steady dose upon afebrile grade 3 neutropenia than group 2 patients who followed the conventional dose modification schedule.
Previous studies have consistently shown that the dose intensity of cytotoxic chemotherapy directly affects the early survival of BC patients [18–20]. Dose intensity was also reported to be important for treatment outcomes in molecular targeted therapy [21]; increasing the dose intensity of fulvestrant significantly enhanced its anti-tumor activity [22]. The landmark trials PALOMA-2 and -3 reported a favorable toxicity profile for palbociclib; however, a considerable number of patients underwent dose reduction or interruption of palbociclib, mainly because of grade ≥ 3 neutropenia [1,23].
In this multicenter retrospective cohort study, we found a significant improvement in PFS in patients who were managed with palbociclib dose maintenance for afebrile grade 3 neutropenia within the first five cycles compared to that in patients with dose delay or reduction. This finding suggests that increasing the palbociclib dose using an aggressive dosing schedule can improve clinical outcomes. Unfortunately, there are no direct pharmacokinetic data to prove that this PFS improvement resulted from higher palbociclib concentration. However, there is indirect evidence of a correlation between neutropenia and palbociclib concentration. Im et al. [24] reported that Asian patients showed more neutropenia events and higher palbociclib trough levels in the PALOMA-2 trial, probably because of their lower body surface area than non-Asian patients (median trough concentration, 92.9 vs. 65.7 ng/mL). Hu et al. [12] also found that palbociclib-induced neutropenia occurred dose-dependently in preclinical models. Moreover, Sun et al. [17] demonstrated a dose-dependent increase in ANC nadir levels in clinical trials of palbociclib. We suggest that a limited dose modification scheme can increase the effective palbociclib concentration, thereby increasing its anti-tumor effect. Further studies are needed to confirm this relationship.
Similar to the results of our previous study, our present findings showed that limited dose modification for neutropenia did not increase hematologic or non-hematologic toxicity. Treatment-related hospitalization events are rare. Comparing our data with those of PALOMA-2, grade 3–4 neutropenia events were found at a similar level in 84.8% of our patients compared to 89.2% in the PALOMA-2 Asian subgroup [24]. The overall AE incidence in group 1 was not different from that in group 2 and the PALOMA-2 Asian subgroup (S5 Table). The median time to the first palbociclib dose reduction was 9 months in group 1 and 56 days in the PALOMA-2 Asian population [24]. Palbociclib dose reduction and delay rates were also lower in group 1 than in the Asian subgroup data of the PALOMA-2 trial (38.5% vs. 56.9% and 29.9% vs. 32.5%, respectively) [24]. Collectively, these data demonstrated that a lower palbociclib dose reduction does not lead to increased clinically significant toxicity.
The PFS benefit hazard ratio of CDK4/6 inhibitors did not vary across major trials in the metastatic setting. However, the recently released phase III PALLAS study results showed no benefit of adjuvant palbociclib therapy in patients with HR-positive early BC. The key explanation for the lack of efficacy is probably the frequent dose interruptions in the palbociclib-treated arm (45.1% within 2 years) [8,10], which were much lower in abemaciclib adjuvant trials [9]. We hope that our new dose modification scheme will enhance the efficacy of palbociclib in an early BC setting, with a higher clinical impact on BC management. The strengths of our study include its large sample size (n=434), homogenously treated population, and balanced demographics among the comparison groups. However, the retrospective nature of our study caused some limitations, including underestimated non-hematologic toxicities, non-uniform visit schedules, and possible selection bias in palbociclib dose modification management. Nevertheless, the most important factor associated with drug exposure was thoroughly reviewed by medical oncologists. This study cohort was combined from four institutions in Korean tertiary referral hospitals with sufficient sample size and follow-up duration to overcome the biases of retrospective analysis. In addition, this study only included Asian patients, who are known to be more vulnerable to neutropenia; therefore, its application to other ethnicities needs further testing.
In conclusion, palbociclib administration without dose interruption and reduction for afebrile grade 3 neutropenia improved PFS outcomes without increasing toxicities in patients with HR+ HER2− mBC. To the best of our knowledge, this is the first real-world study showing the necessity of limited dose modifications to achieve an effective outcome of palbociclib treatment with a safety profile similar to that of the conventional dose modification scheme. A permissive approach to afebrile grade 3 neutropenia and prospective clinical trials for this new dose modification scheme are warranted.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The institutional review boards of each institution approved the study’s protocol. Informed consent was waived, as this study was a retrospective medical record review. Author Contributions Conceived and designed the analysis: Kim SG, Kim MH, Kim GM, Kim JH, Jeong J, Lee J, Jung KH, Sohn J. Collected the data: Kim SG, Kim MH, Kim GM, Kim JH, Kim JY, Park HS, Park S, Park BW, Kim SI, Ji JH, Jeong J, Shin K, Lee J, Kim HD, Jung KH, Sohn J. Contributed data or analysis tools: Kim SG, Kim MH, Park S, Jung KH, Sohn J. Performed the analysis: Kim SG, Kim MH, Park S, Jung KH, Sohn J. Wrote the paper: Kim SG, Kim MH, Jung KH, Sohn J. AcknowledgmentsThis work was supported by Yonsei University College of Medicine [grant number 6-2018-0160]. The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the article for publication.
Fig. 2Cumulative incidence of palbociclib dose reduction in groups 1 and 2 (n=302). Kaplan-Meier curves showing cumulative dose reduction rates over time among group 1 (A) and group 2 (B). Number at risk is shown at the bottom of the graph, with number of censored patients in parentheses. 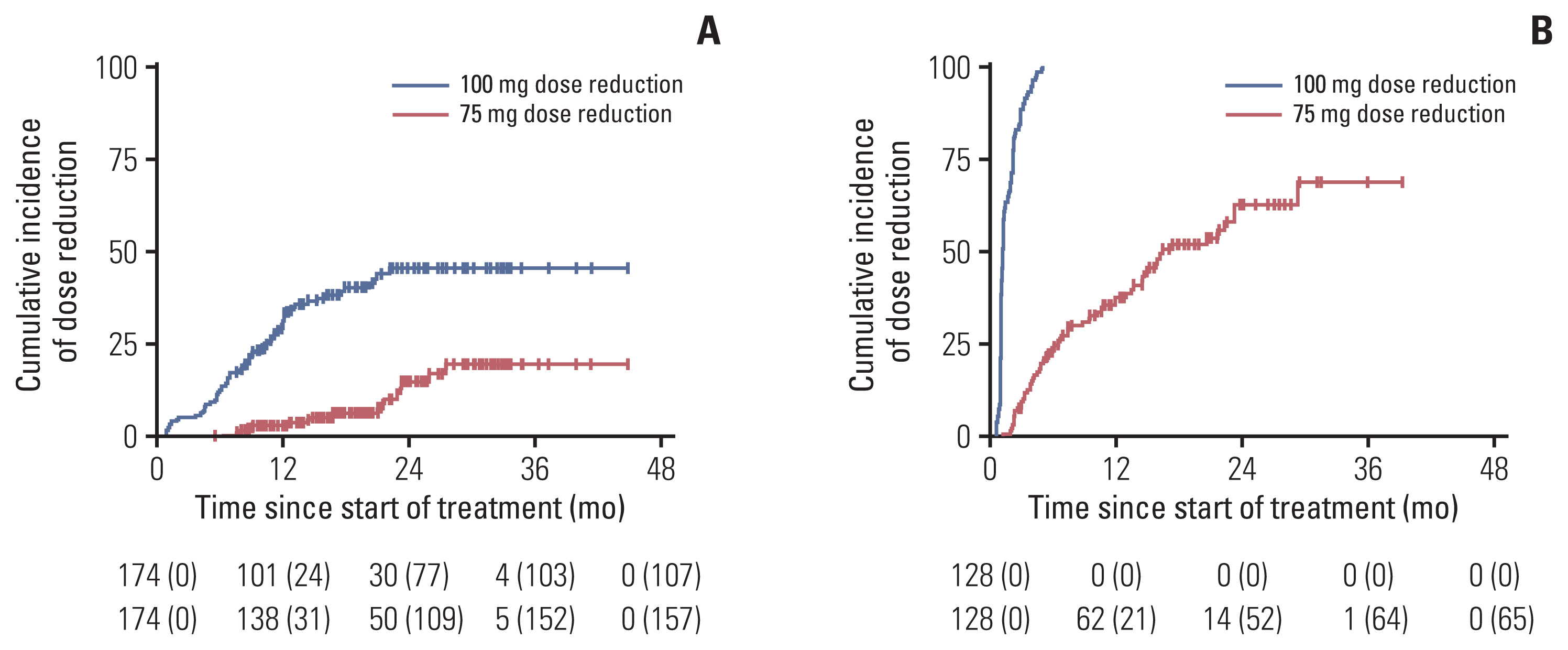
Fig. 3Survival outcomes after dose modification for afebrile grade 3 neutropenia. Progression-free survival (A) and overall survival (B) of group 1 and group 2 patients (n=302). Number at risk is shown at the bottom of the graph, with number of censored patients in parentheses. 
Fig. 4Forest plot of the HR for progression-free survival by subgroup analysis comparing group 1 with group 2 (n=302). CI, confidence interval; HR, hazard ratio. 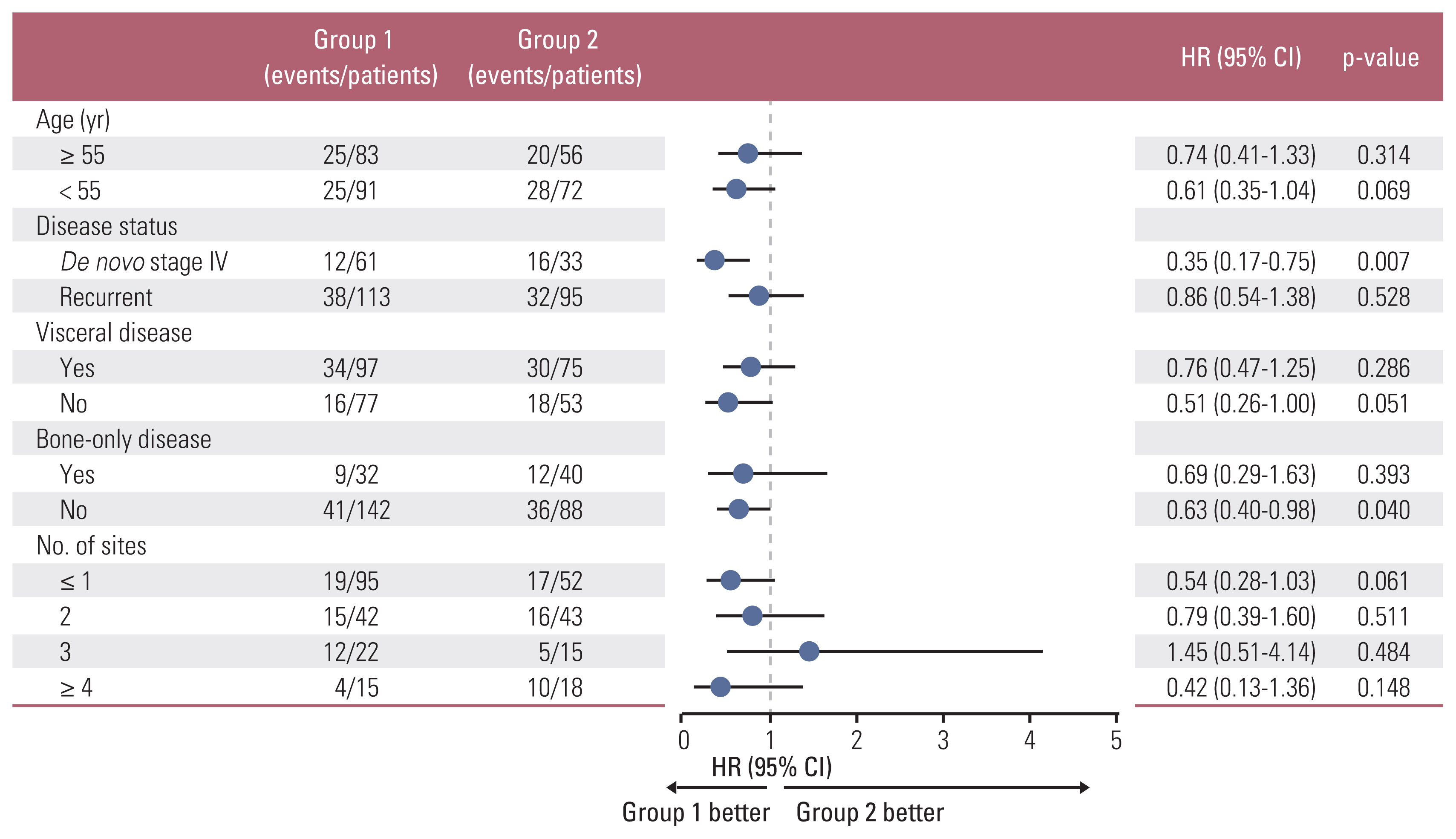
Table 1Limited dose modification scheme for neutropenia
We monitored complete blood cell counts prior to the start of palbociclib therapy and at the beginning of each cycle, as well as on day 15 of the first cycle, and as clinically indicated. For patients who experience a maximum of grade 1 or 2 neutropenia in the first three cycles, we monitored complete blood cell counts for subsequent cycles every 3 months, prior to the beginning of a cycle, and as clinically indicated. Table 2Baseline characteristics of the patient groups according to palbociclib dose scheme
Table 3Palbociclib dose profiles and hospitalization events in patient groups
Table 4Relative dose intensity of palbociclib according to the group Table 5Significance comparison for dose intensity differences
Table 6Univariate and multivariate Cox proportional hazards model of progression-free survival
a) A chi-square test was performed to assess the relationship between the number of sites and visceral disease. The higher the number of metastatic sites, the higher the frequency of visceral disease; the correlation was < 0.0001. Because of the strong linear correlation between the two variables, one variable (i.e., the number of sites) was excluded from the multivariate analysis. Table 7Hematologic and non-hematologic severe adverse events (grade ≥ 3) in study groups References1. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36.
2. Cristofanilli M, Rugo HS, Im SA, Slamon DJ, Harbeck N, Bondarenko I, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC): updated analyses from PALOMA-3. J Clin Oncol. 2021;39(15 Suppl):1000.
3. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48.
4. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–72.
5. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116–24.
6. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46.
7. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–16.
8. Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22:212–22.
9. Johnston SR, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38:3987–98.
10. Cunningham NC, Turner NC. Understanding divergent trial results of adjuvant CDK4/6 inhibitors for early stage breast cancer. Cancer Cell. 2021;39:307–9.
11. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30.
12. Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22:2000–8.
13. Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.
14. Ham A, Kim MH, Kim GM, Kim JH, Kim JY, Park HS, et al. Palbociclib use with grade 3 neutropenia in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183:107–16.
15. IBRANCE (palbociclib). Summary of product characteristics. Sandwich: Pfizer Ltd.; 2018.
16. Berek JS, Matulonis UA, Peen U, Ghatage P, Mahner S, Redondo A, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29:1784–92.
17. Sun W, O’Dwyer PJ, Finn RS, Ruiz-Garcia A, Shapiro GI, Schwartz GK, et al. Characterization of neutropenia in advan-ced cancer patients following palbociclib treatment using a population pharmacokinetic-pharmacodynamic modeling and simulation approach. J Clin Pharmacol. 2017;57:1159–73.
18. Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–11.
19. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440–52.
20. Veitch Z, Khan OF, Tilley D, Tang PA, Ribnikar D, Stewart DA, et al. Impact of cumulative chemotherapy dose on survival with adjuvant FEC-D chemotherapy for breast cancer. J Natl Compr Canc Netw. 2019;17:957–67.
21. Shirotake S, Yasumizu Y, Ito K, Masunaga A, Ito Y, Miyazaki Y, et al. Impact of second-line targeted therapy dose intensity on patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2016;14:e575–83.
22. Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||