INTRODUCTION
Gastric cancer is the second most common cancer in the world and according to the 2002 statistics, gastric cancer is the most prevalent malignancy in Korea (1,2). Surgery remains the only potentially curative treatment, but only 50~60% of gastric cancer patients are surgically resectable and surgery is associated with a high rate of locoregional recurrence. Conventional chemotherapy for treating metastatic gastric cancer remains palliative with only a few patients ever demonstrating long-term survival. However, the rationale for instituting systemic chemotherapy for advanced gastric cancer is based on the results of four randomized trials of chemotherapy versus the best supportive care, and these trials showed a significant improvement for both the median survival time and the quality of life (3~5).
Paclitaxel was isolated from the bark of the Pacific yew tree (Taxus brevifolia) in 1971. Until now, a number of clinical trials have evaluated using paclitaxel as the first-line treatment for metastatic breast, lung, ovarian and digestive cancers, and these trials have confirmed that taxanes are the leading weapons in the armory of cancer treatment (6~9). For stomach cancer, paclitaxel as a single agent or combined with cisplatin has demonstrated promising activity against advanced gastric cancer with the response rate of 17~56% (10~16).
Padexol is formulated in Acephorol 330 as a solubilizer, which is similar to Cremophor EL (polyethoxylated castor oil). The chemical structure and molecular weight of Padexol are the same as those of paclitaxel and the anticancer effects of Padexol are similar to Taxol, as was tested for both on in vitro and in vivo tests.
Therefore, we conducted a multicenter, late phase II trial to evaluate the efficacy and safety of combination chemotherapy of Padexol and cisplatin for patients suffering with advanced gastric cancer, and we compared it with the other previous results of trials with taxane-containing combinations of chemotherapy.
MATERIALS AND METHODS
1) Patient population
The eligible patients were between 18 and 75 years of age, and they were suffering with histologically confirmed metastatic, unresectable locally-advanced or relapsed gastric adenocarcinoma after they had undergone resection. Prior surgery was permitted if this had been done at least 3 weeks before the patient's entry into the study. The other inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, there was at least 1 clinically or radiographically measurable lesion according to the WHO criteria and at least 1 tumor diameter was equal to or greater than 10mm. The patients were required to have nearly normal hematologic values (neutrophils ≥1.5×109/L and a platelet count ≥100×109/L), adequate hepatic function (serum bilirubin ≤1.5 x the upper normal limit (UNL); ALT and AST ≤3.0×UNL or ≤5.0×UNL in the case of liver metastases), adequate renal function (serum creatinine ≤1.5 mg/dl), and they had a life expectancy of at least 3 months.
Patients were ineligible for the study if they had undergone any prior chemotherapeutic regimen unless this treatment had been given in an adjuvant setting at least 24 weeks earlier. Patients were also excluded if they had a history of other carcinomas (except nonmelanoma skin cancer and previously treated in situ cervical cancer), any prior unanticipated severe reaction to the polyethylated caster oil-containing agents, any clinically significant cardiac disease, evidence of CNS metastases, symptomatic peripheral neuropathy of ≥grade 2 on the basis of the National Cancer Institute Common Toxicity Criteria (NCI CTC version 2.0), atrial or ventricular arrhythmia, congestive heart failure, myocardial infarction within 6 months before enrollment, active infections or any other underlying medical condition that would interfere with their participation in the study.
The patients were required to give us a written informed consent before the study-specific procedures were performed, and they were able to comply with the protocol for the duration of the study.
2) Treatment schedule
The treatment consisted of Padexol 175 mg/m2 as a 3-hr infusion, and this was followed by cisplatin 75 mg/m2, which was administered as an IV infusion with a standard hydration method on the first day of each 3-week treatment cycle. The patients were premedicated according to the standard clinical practice with hydrocortisone 100 mg IV, diphenhydramine 50 mg and cimetidine 300 mg IV at 30 min before the administration of paclitaxel for instituting hypersensitivity prophylaxis. Antiemetic therapy was routinely given before each cycle of chemotherapy. The duration of treatment depended on the individual response and tolerance. Those patients with clearly documented progressive disease were discontinued from further study treatment. The patients who responded or whose disease was stable were treated until they finished 6 cycles or the treat ment was stopped if they experienced intolerable toxicity or they withdrew from the study.
3) Dose modifications
The toxicity was evaluated before each treatment cycle according to the NCI CTC version 2.0 guidelines. If the hematopoietic function had not recovered by the first day of the next cycle (neutrophils <1.5×109/L and a platelet count <100×109/L), the administration of paclitaxel and cisplatin was delayed for a maximum of 2 weeks. For the patients that experienced CTC grade 4 neutropenia or thrombocytopenia, grade 3 mucositis or grade 2 peripheral neuropathy, the dosage of paclitaxel and cisplatin was reduced by 20% (paclitaxel 140 mg/m2 and cisplatin 60 mg/m2) for the next cycle. If the above toxicities occurred after the dose reduction, the dose of paclitaxel was then reduced by an additional 20% (paclitaxel 112 mg/m2 and cisplatin 48 mg/m2). Administration of the drugs was discontinued if the patient showed major organ toxicity (including cardiovascular toxicity, nephrotoxicity or hepatotoxicity) of CTC grade ≥3 or other major toxicity (excluding nausea, alopecia and vomiting) of CTC grade ≥3 that was not reversible within 2 weeks after the dose reduction.
4) Study assessments
Physical examination, complete blood counts and the relevant biochemical tests were performed for all the patients before each cycle of therapy. Imaging studies, including computerized tomography, were carried out at the baseline, after every two cycles of therapy and when there was any clinical suspicion of disease progression. Tumor responses were categorized as complete, partial, progression or stable based on the standard WHO criteria. An objective response required one confirmatory follow-up scan at least 4 weeks after the first documentation of the response. Patients who discontinued the study were evaluated at least every 3 months. The patients were considered assessable for their treatment response if they had early disease progression or if they had received at least four cycles of treatment with at least two tumor assessments.
We determined the standard treatment efficacy end points in relation to survival, the objective response, the time to disease progression and the duration of response. The duration of response was defined as the time from the first day of treatment until the first confirmed date of disease progression for the patients who showed a partial or complete response. The time to progression (TTP) was measured from the start of the treatment until disease progression for all the subjects. The overall survival (OS) was measured from the start of the treatment until death.
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTC). The severity of any adverse reaction that was not defined by the NCI CTC was graded as 1, mild; 2, moderate; 3, severe; and 4, life- threatening.
5) Statistical analysis
The trial was conducted according to the group sequential design that was suggested by Chang et al. with the response rate as the primary end point (17). The number of patients required for the study was calculated based on a targeted activity level of 37% and a minimum activity level of 20%, with α error of 0.05 and β error of 0.20; therefore, the required number of patients was 50. The interim analysis was carried out when the first 30 assessable patients had been recruited. The trial would be evaluated if 11 of more responses were observed with the conclusion that the regimen was sufficiently active to be submitted for further study.
The analysis of the clinical efficacy was performed on a per protocol basis and according to an intent-to-treat analysis. Kaplan-Meier estimates were used in the analysis of all the time-to-event variables (duration of response, time to progression and overall survival), and the 95% confidence interval (CI) for the median time to the event was computed. The SPSS program (SPSS, Inc, Chicago, IL) for Windows was used for the statistical computation.
RESULTS
From August 2003 to September 2004, 39 patients at 5 hospitals were enrolled onto the study. All the patients received the test drug for more than one cycle. All the patients were assessable for the safety analysis and 34 patients were assessable for their response. 5 patients were excluded from the response analysis: one patient expired from an unknown cause after one cycle. One patient expired due to gastrointestinal bleeding after one cycle. Two patients refused further treatment after the first and second cycle, respectively. One patient was taken off the study because of adverse events related to the treatment (nephrotoxicity).
1) Patient demographics
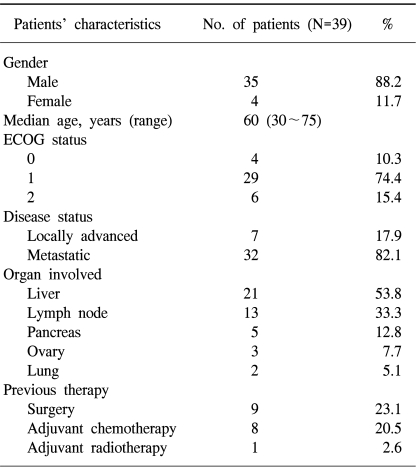
The patient characteristics are listed in Table 1. The median patient age at the study's entry point was 60 years (range: 30~75 years), and most of the study population was male (35 out of 39). The patients weighed from 40 to 76 kg (median weight: 59 kg). 85% of the patients had a performance status of 0 to 1. Two patients had local advanced disease that was surgically unresectable. 5 patients had pancreatic invasion and 32 patients had metastatic disease (liver: n=21; lymph node: n=13; ovary: n=3; lung: n=2). 9 patients (23%) had previously undergone surgery with a curative intent for gastric cancer and 8 patients (21%) had previously received 5FU-based adjuvant chemotherapy.
2) Treatment administration
A total of 161 treatment cycles were administered with a median of 4 cycles per patient (range: one to six cycles). 17 of the patients received the full 6 cycles of chemotherapy and 25 patients (64%) received at least 4 cycles. The dose of paclitaxel was reduced in 31 courses and the dose of cisplatin was reduced in 30 courses. Thus, the actual administered dose of paclitaxel was 56.32 mg/m2/week and that of cisplatin was 24.2 mg/m2/week, and this corresponded to 96.5% and 96.8% of the planned doses, respectively. Neutropenia, hepatotoxicity and neuropathy were the common reasons for the dose reduction of paclitaxel.
3) Efficacy and survival
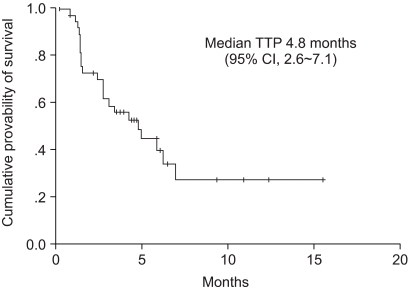
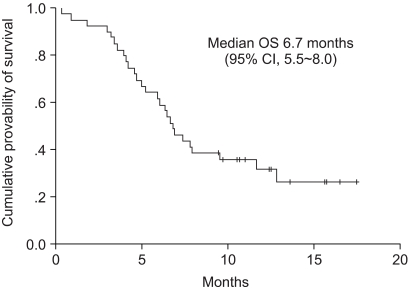
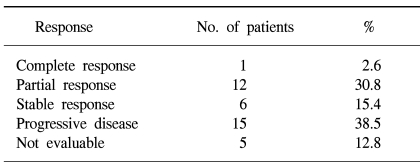
Among the 39 patients who received the test drugs, 34 patients were evaluable for response. Of these, 13 responses were observed and the response rate met the early stoppage rules. On the intent-to-treat analysis, 1 patient (3%) achieved CR, 12 patients (31%) achieved PR, 6 patients (15%) had SD and 15 patients (38%) had progressed; therefore, the overall response rate was 33% (95% CI: 18.5% to 48.1%)(Table 2). Among the 34 response-assessable patients, the response rate was 38% (95% CI: 22% to 55%). For the 13 responders, the median duration of response was 7.1 months. The median follow-up duration was 7.1 months (range: 1.8 to 17.5 months). The median time to progression (TTP) for all the patients was 4.8 months (95% CI: 2.6 to 7.1 months). The median survival time on the intent-to-treat analysis was 6.7 months (95% CI: 5.5 to 8.0 months). The Kaplan-Meier estimate of survival is shown in Fig. 1 and 2. The 1-year survival rate was 32%.
4) Safety
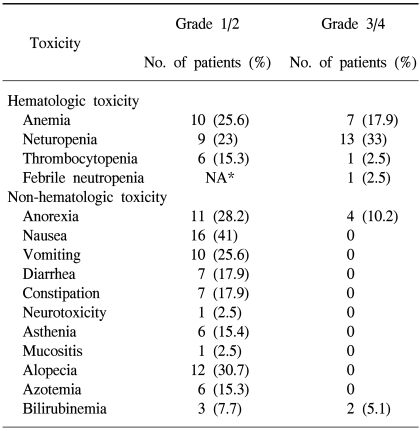
All the registered patients were assessable for their treatment-related adverse events and toxicities that were observed during the study, and these are listed in Table 3. The most common grade 3 or 4 toxicities were neutropenia (33%) and anemia (15%). The non-hematologic toxicity was mild and there were 12 patients (30.7%) with alopecia, 4 patients (10%) with grade 3 anorexia and 2 patients (5%) with grade 3 or 4 bilirubinemia. 5 patients died during the study period. 3 patients died in relation to the gastric cancer itself: 2 died of disease progression and 1 died from acute gastrointestinal bleeding. None of them had adverse events of over grade 3 from the chemotherapy. The others died irrespective of the disease: a patient died of nephrotoxicity with generalized edema and we considered that this was probably related with the cisplatin. The other patient died from aspiration pneumonia.
DISCUSSION
While instituting chemotherapy for gastric cancer is largely palliative, a number of clinical trials have established the role of chemotherapy for the treatment of patients with advanced gastric cancer. Several investigators have attempted to improve on the relatively low response rate and the poor survival rate by using 5-FU alone or by combining 5-FU with other cytotoxic agents (18,19). Therefore, cisplatin and fluorouracil-based therapy with or without additional epirubicin has been considered the reference regimen for advanced gastric cancer for many years. However, the chemotherapy administered in the "old days" was limited by a low complete response rate, a response duration that was short-lived and the considerable toxicities. Within the last decade, several new drugs have emerged, including taxanes, irinotecan, oxaliplatin and oral fluropyrimidines, and these drugs provide for more effective and better tolerated regimens for the treatment of advanced gastric cancer (10~16,20~23).
Paclitaxel has shown encouraging activity for the treatment of patients with advanced gastric cancer and it appears to have a schedule-dependent synergy with the platinum compounds; this has been documented in the human gastric cancer cell lines (18). Ajani et al. have reported on the activity of paclitaxel as a single agent for the treatment of advanced gastric cancer as given in either a 3-hour or 24-hour infusion. The response rates were 17% and 20%, respectively, with the median duration of response being 6.5 months (10). Until now, several phase II trials that have evaluated the efficacy of paclitaxel-containing combinations with 5FU and/or platinum have recently reported encouraging response rates that ranged from 17~60% in the first and second line settings (10~16).
In this phase II study, we report on the efficacy and safety of a combination of Padexol and cisplatin for the treatment of advanced gastric cancer, with an objective response rate, a median time to progression and a median overall survival of 33%, 4.8 months and 6.7 months, respectively. The results of a recent study with Genexol (another paclitaxel formulation) and cisplatin for advanced gastric cancer has been reported by Park et al (16). When we consider that our study included more elderly patients who had a relatively poor performance status (ECOG 2) and a few early deaths that were unrelated with disease, the outcome of the Padexol and cisplatin combination therapy was similar to the results of other studies. The current combination of Padexol and cisplatin was well tolerated; the major toxicity was grade 3 or 4 neutropenia, and this occurred in 33% of the patients. It's notable that no hypersensitivity reactions and serious neurotoxicity were observed.
CONCLUSIONS
We suggest that the combination of paclitaxel and cisplatin is an effective and safe treatment for advanced gastric cancer and it offers a modest benefit in terms of palliation and survival. However, well-controlled randomized trials need to be designed to confirm the superiority of this treatment regimen as compared to the other regimens.