AbstractPurposeThe impact of epidermal growth factor receptor (EGFR) mutation in locally advanced non–small cell lung cancer (NSCLC) remains controversial. This study was conducted to investigate the clinical outcomes and recurrence patterns after definitive chemoradiotherapy (CRT) in patients with unresectable stage III non-squamous-cell lung cancer according to EGFR mutation status.
Materials and MethodsWe retrospectively reviewed 604 patients with pathologically confirmed stage III NSCLC who were treated with definitive CRT and were examined for EGFR mutation at Samsung Medical Center, Korea, from January 2013 to December 2018. Among them, we identified 236 patients with stage III non-squamous-cell lung cancer who were treated with definitive CRT and were examined for EGFR mutation status. We analyzed the frequency of EGFR mutation, progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and recurrence pattern.
ResultsAmong 236 patients, EGFR mutation was detected in 71 patients (30.1%) and the median follow-up duration was 41.7 months. There were no significant differences in PFS (9.9 vs. 10.9 months, p=0.236), and ORR to CRT (93.0% vs. 90.3%, p=0.623) according to EGFR mutation status. However, the EGFR mutant group showed significantly higher recurrence (88.7% vs. 75.2%, p=0.022), distant metastasis (76.1% vs. 61.2%, p=0.036) rates, especially brain (38.0% vs. 12.7%, p < 0.001), and better median OS (59.2 vs. 41.3 months, p=0.037) compared with patients without EGFR mutation.
IntroductionApproximately 85% of lung cancer patients have non–small cell lung cancer (NSCLC) and about one-third of patients with NSCLC present with locally advanced NSCLC [1,2]. The majority of locally advanced NSCLC patients have unresectable disease or extensive mediastinal lymphadenopathy. Concurrent chemoradiotherapy (CRT) is the standard treatment for these patients [3,4].
Recently, the PACIFIC trial demonstrated that consolidation with durvalumab following CRT, in unresectable stage III NSCLC patients whose disease has not progressed, significantly prolonged progression-free survival (PFS) and overall survival (OS) [5]. Therefore, the current standard treatment is CRT followed by durvalumab. Despite the good outcomes of the new treatment strategy, the majority of patients treated with CRT followed by durvalumab developed disease progression and died [6]. Therefore, there is a need for a predictive biomarker that appropriately selects the best treatment for patients individually.
The findings of driver mutations in metastatic NSCLC patients, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene translocation, brought paradigm shifts in the therapeutic strategy for metastatic NSCLC [7,8]. However, there have been no prospective studies and only a few retrospective studies are available that reported an association of EGFR mutation with clinical outcomes including PFS, OS, and recurrent patterns in locally advanced NSCLC patients treated with CRT [9–12]. Given the small sample sizes and heterogeneous patient populations, the role of EGFR mutation in locally advanced NSCLC remains controversial. In this study, we investigated the frequency of EGFR mutations and clinical outcomes in patients with unresectable stage III non-squamous-cell lung cancer after definitive CRT according to EGFR mutation status.
Materials and Methods1. PatientsWe retrospectively reviewed 604 patients with pathologically confirmed stage III NSCLC who were treated with definitive CRT and were examined for EGFR mutations at Samsung Medical Center, Korea, from January 2013 to December 2018. NSCLC stage evaluation was based on the American Joint Committee on Cancer 8th Edition Cancer Staging Manual. A total of 262 patients diagnosed with squamous cell carcinoma were excluded from the analysis. Among 342 remaining patients, 102 who were treated with definitive CRT due to metachronous oligoreccurrence after surgical resection and four who did not complete planned radiation were also excluded (Fig. 1). The baseline clinicopathologic characteristics were reviewed with their medical records. All EGFR mutational status was analyzed from tumor specimens using a peptide nucleic acid clamp kit and polymerase chain reaction-based methods at the time of first diagnosis. The Institutional Review Board (IRB No. 2022-06-022) at the Samsung Medical Center approved this study and the need for informed consent was waived because of the retrospective nature of this study.
2. Treatment and outcomesAll 236 patients received 60–74 Gy of thoracic radiation therapy concurrently with platinum doublet chemotherapy. Chemotherapy regimens included paclitaxel plus cisplatin, paclitaxel plus carboplatin, docetaxel plus cisplatin, etoposide plus cisplatin, and etoposide plus carboplatin. Among 236 patients, seven received consolidation with durvalumab following CRT. All patients were evaluated radiologically for clinical outcomes including objective response rate (ORR), disease control rate (DCR), PFS, and OS according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 through computed tomography (CT) or magnetic resonance imaging (MRI). The first follow-up and response evaluation were scheduled 1 month after completion of CRT with chest CT scan. Subsequent follow-up evaluations were conducted at 3–4-month intervals thereafter. The brain MRI was performed only when clinical signs and symptoms suggestive of possible brain involvement were present. The recurrence was evaluated radiologically and defined as documentation of disease progression. PFS was defined as the time from CRT start date until the date of documented disease progression or death from any cause. OS was defined as the time from the CRT start date until death from any cause. According to the RECIST, ORR was defined as the proportion of patients with a complete response (CR) or partial response (PR) to treatment, and DCR was defined as the proportion of patients with a CR, PR, or stable disease to treatment.
3. Statistical analysesThe cutoff date for data collection was December 31, 2021. Descriptive statistics were used to summarize patient and tumor characteristics and treatment history and were reported as proportions and medians. Data are presented as numbers (%) for categorical variables. Correlations of response rates and recurrence patterns according to EGFR mutation status were analyzed by Fisher’s exact test. Survival analyses were performed with the Kaplan-Meier method, and differences were analyzed with the log-rank test. Hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) were calculated with the Cox proportional hazards model. All p-values were two-sided, and statistical significance was set at p < 0.05. Univariate analysis of prognostic factors was performed using Cox proportional hazards models for PFS and OS. Multivariate analysis was used in factors that were significant (p < 0.05) on univariate analysis. Statistical analyses were performed with the Statistical Package for the Social Sciences software program ver. 25 (IBM Corp., Armonk, NY).
Results1. Patient characteristicsAll 236 patients were retrospectively reviewed in this study. EGFR mutation test was conducted for all patients. EGFR mutation was detected in 71 patients (30.1%). The most frequent type of EGFR mutation was a deletion in exon 19 (37 patients, 52.1%). L858R point mutation was detected in 23 patients (32.4%) and uncommon mutations (exon 20 insertion, G719X, S768I, T790M) were detected in 11 patients (15.5%).
Table 1 presents the clinical characteristics of patients with and without EGFR mutation. All patients received 60–74 Gy of radiation therapy concurrently with platinum doublet chemotherapy. The EGFR mutation group had a statistically higher proportion of females (53.5%), never smokers (62.0%), low T category (77.5%), high N category (93.0%), and adenocarcinoma (100.0%) (p < 0.05).
2. SurvivalThe median follow-up duration was 41.7 months (2.8 to 106.3 months). Overall, 140 patients died and 204 patients experienced disease progression. Median PFS was 9.9 months for the EGFR mutation versus 10.9 months for EGFR wild type. Median OS was 59.2 months for the EGFR mutation versus 41.3 months for EGFR wild type. There was no statistically significant difference in PFS according to EGFR mutation status (p=0.236) (Fig. 2A). There was a statistically significant difference in OS according to EGFR mutation status (p=0.037) (Fig. 2B). The 52 patients (82.5%) with EGFR mutation were treated with EGFR tyrosine kinase inhibitors (TKIs) (gefitinib, n=19; erlotinib, n=7; afatinib, n=25; olmutinib, n=1) after recurrence. On the other hand, most patients (77/124, 62.1%) with EGFR wild-type were treated with cytotoxic chemotherapy. Also, those patients (10/124, 8.1%) in the EGFR-wild type and ALK-positive were treated with ALK TKIs based on the driver gene mutation after recurrence.
Among 71 patients harboring EGFR mutations, we analyzed survival according to the EGFR mutation subtype, but there were no statistically significant differences in PFS and OS according to EGFR mutation subtype (p=0.204 and p=0.205, respectively) (Fig. 3A and B). We additionally conducted survival analyses for PFS and OS according to EGFR mutation. EGFR mutation also was not an independent factor in univariate analysis for PFS (p=0.237) (Table 2). Univariate analysis for OS revealed age (p=0.031), and EGFR mutation (p=0.039) as significant factors (Table 3). Multivariate analysis for OS showed age (p=0.037), and EGFR mutation (p=0.045) as significant independent prognostic factors (Table 3).
3. Response to CRTWe compared the tumor response to CRT according to EGFR mutation status. ORR and DCR were 93.0% and 97.2%, respectively, in patients with EGFR mutation and 90.3% and 93.3%, respectively, in patients with wild-type EGFR. ORR and DCR according to EGFR mutation status were not significantly different (p=0.623 and p=0.354, respectively) (Table 4).
4. Recurrence patterns
Table 5 shows the initial recurrence patterns according to EGFR mutation status. Among 71 patients with EGFR mutation, 63 (88.7%) developed tumor recurrence without death and 54 patients (76.1%) had distant metastasis. Among 165 patients with wild-type EGFR, 124 patients (75.2%) developed tumor recurrence without death and 101 patients (61.2%) had distant metastasis.
The EGFR mutant group showed significantly higher recurrence rates (88.7% vs. 75.2%, p=0.022) and distant metastasis rates (76.1% vs. 61.2%, p=0.036) compared with the EGFR wild-type group. In contrast, loco-regional recurrence incidence was not significantly different according to EGFR mutation status (p > 0.99). The most common distant metastasis site in the EGFR mutant group was the brain (38.0%) followed by lung-to-lung (36.6%), bone (14.1%), pleura (8.5%), liver (5.6%), distant lymph node (4.2%), and adrenal gland (4.2%). The EGFR mutant group showed a significantly higher incidence of brain metastasis compared with the EGFR wild-type group (38.0% vs. 12.7%, p < 0.001).
DiscussionIn our study, we identified the frequency of EGFR mutation and the clinical outcomes in patients with stage III non-squamous-cell lung cancer after definitive CRT according to EGFR mutation status. We found that 30% of patients with unresectable stage III non-squamous-cell lung cancer treated with CRT harbor an EGFR mutation, which is consistent with previous studies [9,10,12]. Intriguingly, EGFR mutation was associated with higher recurrence rates and distant metastasis, especially in the brain, compared with patients without EGFR mutation. The association between EGFR mutation and brain metastasis from metastatic NSCLC has been reported in previous studies. These studies have consistently reported that the risk of brain metastasis increased in metastatic NSCLC with EGFR mutation [12–14]. Based on these results, EGFR mutation may impact the pattern of distant metastasis. Despite higher recurrence rates and distant metastasis, the OS was significantly higher in patients with EGFR mutation than patients with EGFR wild-type. After recurrence or disease progression, most patients (82.5%) with EGFR mutation were treated with EGFR TKI. On the other hand, most patients (62.1%) with EGFR wild-type were treated with cytotoxic chemotherapy and those patients (8.1%) in the EGFR-wild type and ALK-positive were treated with ALK TKIs. Therefore, the dissociation between the high recurrence rate and OS could be due to the response to salvage treatment based on the driver gene mutation after recurrence.
The impact of EGFR mutation on clinical outcomes after definitive CRT in stage III NSCLC remains controversial. Nakamura et al. [10] and Yagishita et al. [11] reported no significant difference in PFS according to EGFR mutation status. Alternatively, Park et al. [9] and Tanaka et al. [12] reported significantly shorter PFS in patients with EGFR mutation compared with the wild-type EGFR. These different results indicate that the impact of EGFR mutation on PFS after CRT remains unestablished. However, these studies have consistently demonstrated that the incidence of distant metastasis and disease progression is high and that the brain was the most frequent site of distant metastasis in patients with EGFR mutation, which were consistent with our results. The high incidence of recurrence with distant metastases, including those in the central nervous system (CNS), highlights the need for regular brain MRI follow-up and CNS-penetrant targeted therapy as consolidation in patients with EGFR mutation.
Several clinical trials of EGFR TKIs in unresectable stage III EGFR-mutated NSCLC are evaluated [15,16]. These phase-II studies reported that gefitinib and erlotinib showed improved clinical outcomes, however, they have less penetration of the blood-brain barrier and lower CNS activity than osimertinib. Also, these studies did not include regular brain image follow-up.
Recently, the ADAURA phase III trial demonstrated that osimertinib, a CNS active third-generation EGFR TKI, significantly improved disease-free survival in patients with completely resected, EGFR-mutated stage II to -IIIA NSCLC after completing standard adjuvant chemotherapy (HR, 0.17; 95% CI, 0.1 to 10.26). Furthermore, adjuvant osimertinib treatment was associated with a marked reduction in CNS recurrence or death (HR, 0.18; 95% CI, 0.10 to 0.33) [17]. These results suggest that osimertinib might be a good candidate for consolidation therapy following CRT in patients with locally advanced EGFR-mutated NSCLC. Currently, the phase-III LAURA trial is underway to assess the efficacy and safety of consolidation with osimertinib following CRT in patients with stage III unresectable EGFR-mutated NSCLC [18]. This included patients with locally advanced, unresectable stage III EGFR-mutated NSCLC after completing CRT who were randomly assigned to receive osimertinib or placebo until disease progression. The primary endpoint of the study was PFS in patients with locally advanced, unresectable stage III EGFR-mutated NSCLC. Secondary endpoints include time to CNS PFS and cumulative incidence at 12 and 24 months, OS, PFS by mutation status, time to death or distant metastasis, and safety. How well osimertinib consolidation can reduce recurrence rate, especially brain metastasis will be an important insight from the LAURA trial. Our results provide a strong rationale to investigate CNS-penetrant EGFR TKI as consolidation in unresectable locally advanced NSCLC with EGFR mutation.
In the era of immune checkpoint inhibitors (ICI), the PACI-FIC trial demonstrated that consolidation with durvalumab following CRT significantly improved clinical outcomes in unresectable stage III NSCLC patients whose disease has not progressed. However, the PACIFIC trial subgroup analysis of prognostic factors for PFS showed that patients with EGFR mutation may benefit less from CRT followed by durvalumab (HR, 0.84; 95% CI, 0.40 to 1.75) [5,6]. Also, a meta-analysis demonstrated that patients with EGFR-mutated metastatic NSCLS exhibited poor survival when treated with ICIs [19], but the reason is not clear. Previous studies have suggested that EGFR mutant tumors generate an immunosuppressive tumor microenvironment with less programmed death-ligand 1 expression, reduced tumor mutational burden and neoantigen presentation, and decreased tumor infiltrating lymphocyte infiltration, all of which decrease ICI efficacy [20].
The optimal treatment in unresectable stage III EGFR-mutated NSCLC patients remains unclear. Results with EGFR TKIs showed improved clinical outcomes when given concurrently or induction to CRT in unresectable stage III EGFR-mutated NSCLC patients [11,16]. However, they should be confirmed in the phase III trial. Also, in the PACIFIC trial, only 6% of patients had EGFR mutation [5]. Given the post-hoc analysis and small sample size, further evaluation is needed to evaluate the role of durvalumab consolidation in EGFR-mutant patients. Currently, the phase III LAURA trial is ongoing. This study will evaluate the role of EGFR TKI to improve clinical outcomes and suggest a new treatment strategy, which is the definitive CRT followed by EGFR TKI.
This study has several limitations. First, it is a single-center retrospective study, which can lead to bias. Second, only Asian patients with NSCLC were analyzed, and this limits its generalizability because of differences in molecular profiles and clinical features between Western and Eastern patients with NSCLC. Nevertheless, to the best of our knowledge, this study is one of the largest with a long-term follow-up analysis of the impact of EGFR mutation in patients with unresectable stage III non-squamous-cell lung cancer after definitive CRT.
In conclusion, patients with EGFR mutation–positive unresectable stage III non-squamous lung cancer exhibited higher recurrence and distant metastasis rates, especially in the brain.
NotesEthical Statement This study was approved by the Institutional Review Board (IRB No. 2022-06-022) at Samsung Medical Center and the need for individual consent for this retrospective analysis was waived. Author Contributions Conceived and designed the analysis: Kim H, Ahn MJ. Collected the data: Kim H, Park S, Jung HA, Sun JM, Lee SH, Ahn JS, Ahn MJ. Contributed data or analysis tools: Kim H, Park S, Jung HA, Sun JM, Lee SH, Ahn JS, Ahn MJ. Performed the analysis: Kim H, Ahn MJ. Wrote the paper: Kim H, Ahn MJ. Fig. 1Flow diagram of the study population. CRT, chemoradiotherapy; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer. 
Fig. 2Kaplan-Meier curves of progression-free survival (A) and overall survival (B) according to epidermal growth factor receptor (EGFR) mutation status. CI, confidence interval. 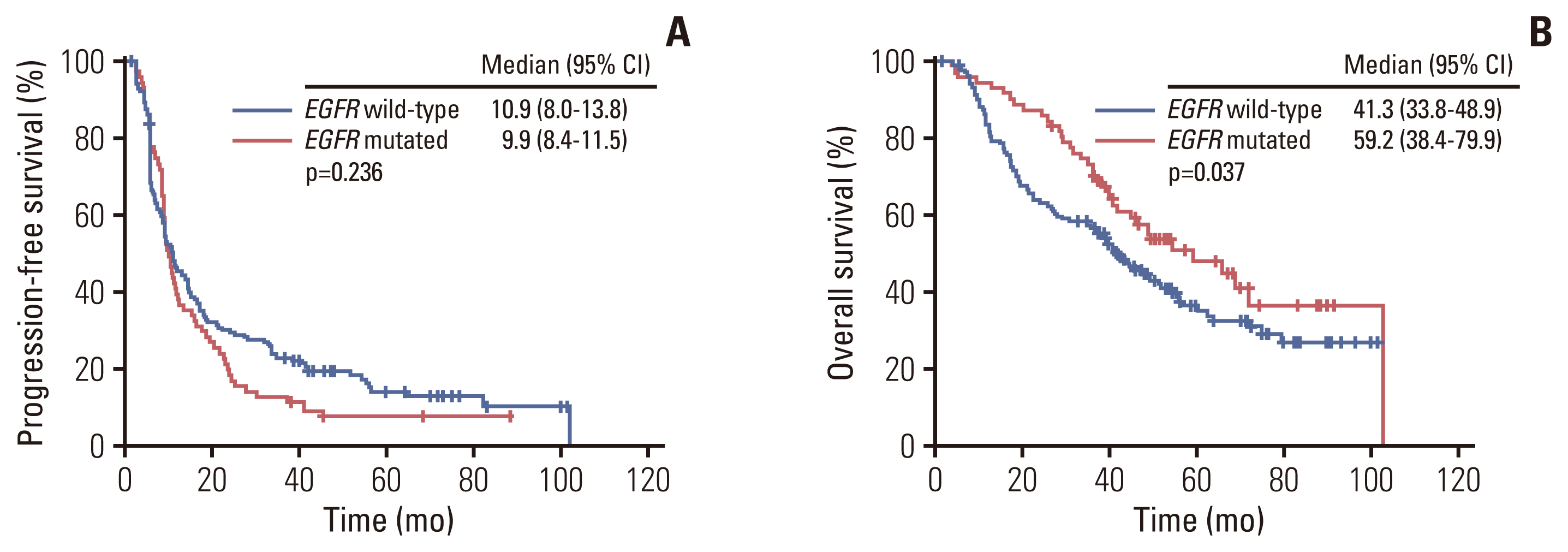
Fig. 3Kaplan-Meier survival curves of progression-free survival (A) and overall survival (B) according to epidermal growth factor receptor (EGFR) mutation subtypes. CI, confidence interval. 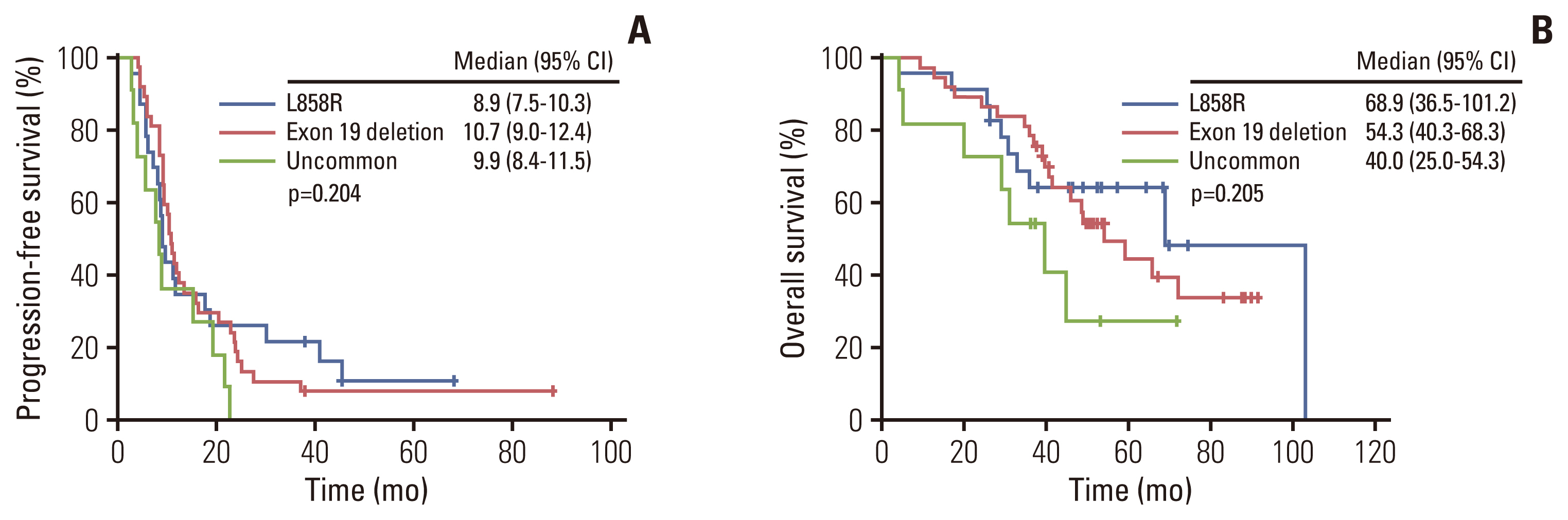
Table 1Patient characteristics Table 2Univariate analysis of progression-free survival Table 3Univariate and multivariate analysis of overall survival Table 4Response rate according to EGFR mutation Table 5Initial recurrence pattern according to EGFR mutation References1. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
3. Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90.
4. Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol. 2007;25:4146–52.
5. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
6. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC: an update from the PACIFIC trial. J Thorac Oncol. 2021;16:860–7.
7. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
8. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703.
9. Park SE, Noh JM, Kim YJ, Lee HS, Cho JH, Lim SW, et al. EGFR mutation is associated with short progression-free survival in patients with stage III non-squamous cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. 2019;51:493–501.
10. Nakamura M, Kageyama SI, Niho S, Okumura M, Hojo H, Motegi A, et al. Impact of EGFR mutation and ALK translocation on recurrence pattern after definitive chemoradiotherapy for inoperable stage III non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2019;20:e256–64.
11. Yagishita S, Horinouchi H, Katsui Taniyama T, Nakamichi S, Kitazono S, Mizugaki H, et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91:140–8.
12. Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J Thorac Oncol. 2015;10:1720–5.
13. Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111:1–10.
14. Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9:195–9.
15. Komaki R, Allen PK, Wei X, Blumenschein GR, Tang X, Lee JJ, et al. Adding erlotinib to chemoradiation improves overall survival but not progression-free survival in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;92:317–24.
16. Hotta K, Saeki S, Yamaguchi M, Harada D, Bessho A, Tanaka K, et al. Gefitinib induction followed by chemoradiotherapy in EGFR-mutant, locally advanced non-small-cell lung cancer: LOGIK0902/OLCSG0905 phase II study. ESMO Open. 2021;6:100191.
17. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–23.
18. Lu S, Casarini I, Kato T, Cobo M, Ozguroglu M, Hodge R, et al. Osimertinib maintenance after definitive chemoradiation in patients with unresectable EGFR mutation positive stage III non-small-cell lung cancer: LAURA trial in progress. Clin Lung Cancer. 2021;22:371–5.
|
|
|||||||||||||||||||||||||||||||||||||||