AbstractLeptomeningeal metastasis (LM) is a rare but fatal clinical condition with a short survival time. The incidence of LM from epidermal growth factor receptor mutant (EGFRm) non–small cell lung cancer (NSCLC) has increased due to the limited efficacy of first- or second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in the central nervous system (CNS). Osimertinib is a third-generation, irreversible, CNS penetrant, oral EGFR TKI that demonstrates promising efficacy in CNS metastases regardless of T790M. Herein, we report four cases of T790M-negative EGFRm NSCLC patients treated with osimertinib combined with systemic chemotherapy, who progressed on prior EGFR TKI and developed LM with extracranial lesions. The combination treatment was well tolerated, and the mean overall survival from LM diagnosis was 14.7 months (95% confidence interval, 10.4 to 19.0). These results suggest that osimertinib combined with systemic chemotherapy would be a reasonable treatment option for T790M-negative EGFRm NSCLC patients who develop LM with extracranial progression to prior EGFR TKI. A further prospective study is warranted.
IntroductionThe central nervous system (CNS) is a sanctuary site for tumor cells and therapeutic agents due to the transport restriction caused by the blood-brain barrier (BBB). Malignant cells metastasize to the leptomeningeal space through dissemination and via endo-neural and peri-neural spread [1]. The incidence of leptomeningeal metastasis (LM) from non-small cell lung cancer (NSCLC) has been estimated to be 3%–4%, which increases to 9%–16% in patients with epidermal growth factor receptor mutant (EGFRm) NSCLC owing to improved survival from new target agents and limited efficacy of first- or second-generation EGFR tyrosine kinase inhibitors (TKIs) [2,3]. LM has a detrimental effect on the quality of life in patients and is associated with a very poor prognosis. The efficacy of conventional therapies, such as whole-brain radiation therapy (WBRT) and intrathecal (IT) chemotherapy, has not been established in previous trials [4,5]. The median overall survival (OS) of patients with LM is three to 10 months from LM diagnosis even with treatment [2,6].
A previous retrospective study showed that, in patients with LM from EGFRm NSCLC, those treated with EGFR TKIs demonstrated longer OS than those who did not [2]. Erlotinib and gefitinib are first-generation EGFR TKIs, and erlotinib was more effective than gefitinib against LM [7]. Osimertinib, a third-generation, irreversible, CNS penetrant, oral EGFR TKI, has been the standard first- or second-line therapy in EGFR T790M-mutant NSCLC [8]. Given the high CNS penetration efficacy, osimertinib has been investigated in patients with LM. In the BLOOM study, osimertinib 160 mg once daily showed a clinically meaningful benefit in EGFRm NSCLC with LM. The median OS from LM was 11.0 months (95% confidence interval [CI], 8.0 to 18.0) [9]. In the AURA study, the patients treated with osimertinib 80 mg once daily for LM from EGFR T790M-positive NSCLC tolerated it well and achieved a 55% objective response rate with a median OS of 18.0 months (95% CI, 6.3 to not reached) [10]. In another study, osimertinib showed survival improvement in patients with LM from EGFRm NSCLC regardless of T790M mutation (10.1 months [95% CI, 4.3 to 15.8] in T790M-positive patients vs. 9.0 months [95% CI, 6.8 to 11.2] in T790M-negative patients, p=0.936) [11].
However, there was no consensus therapy for EGFRm NSCLC patients with LM and extracranial progression who had been resistant to previous EGFR TKI without EGFR T790M mutation. Here, we report four cases of T790M-negative EGFRm NSCLC patients treated with osimertinib combined with systemic chemotherapy who progressed on prior EGFR TKI and developed LM along with extracranial lesions.
Case Report1. Case 1A 48-year-old female was a never-smoker and had a history of pulmonary tuberculosis cured by medication. The pulmonary nodule was found on chest X-ray, and NSCLC (adenocarcinoma) with brain and bone metastases was diagnosed in further evaluation in January 2017. She underwent gamma-knife surgery (GKS) for metastatic brain lesion and radiotherapy for pelvic and spinal bone metastases, followed by gefitinib because EGFR L858R mutation was identified. After 15 months, disease progression was noted, and pemetrexed plus cisplatin were administered as second-line therapy because there was no T790M mutation upon repeat biopsy. However, in August 2020, the patient complained of a headache, and spinal tap revealed increased intracranial pressure (IICP) and was positive for craniospinal fluid (CSF) cytology. To control IICP, a ventricle-peritoneal (VP) shunt was inserted. Given the newly developed LM and progression of extracranial disease, 80 mg of osimertinib once daily was started for LM; and bevacizumab, docetaxel, and carboplatin were administered for extracranial disease. Since then, the patient has continued combination therapy for 9.6 months without progression of LM or extracranial disease. The patient complained of general weakness of grade 1 without other adverse events. However, eventually, the patient died of pneumonia progressive to septic shock in January 2021. The brain magnetic resonance images (MRI) before and after osimertinib administration are presented in Fig. 1A.
2. Case 2A 57-year-old, never-smoker female was diagnosed with NSCLC with EGFR exon 19 deletion in January 2018. She had multiple brain metastases and disseminated lung to lung metastases at the time of the first diagnosis. After 17 months of gefitinib as first-line therapy, progression was noted without T790M mutation and treated with a combination of atezolizumab, bevacizumab, paclitaxel, and carboplatin. In January 2021, follow-up brain MRI demonstrated LM without clinical symptoms, but CSF cytology was positive. Thus, pemetrexed and carboplatin combined with 80 mg osimertinib once daily were started for extracranial disease and LM, respectively. The patient suffered diarrhea of grade 2, leading to a dose reduction of osimertinib to 80 mg every other day. After changing the medication schedule, diarrhea improved. LM and systemic disease have been well controlled for 6 months. Chest computed tomography (CT) and brain MRI before and after osimertinib administration are presented in Fig. 1B.
3. Case 3A 58-year-old, never-smoker female visited the emergency room with dyspnea in January 2020. Bilateral pleural and pericardial effusions were identified on chest CT, and NSCLC was diagnosed in further evaluation. EGFR L858R mutation was detected in pericardial fluid analysis, and gefitinib was started. After 6 months, the patient had a seizure, a metastatic brain lesion and LM were suggested in brain MRI, and CSF cytology was positive. A metastatic mass in the brain was treated with GKS, and the Ommaya reservoir was inserted, via which IT methotrexate (IT-MTX) was administered for LM. An EGFR T790M mutation was not detected in cell-free DNA. Because there was no response to IT-MTX, 80 mg of osimertinib once daily for LM and systemic chemotherapy with pemetrexed and cisplatin was initiated for extracranial disease. The patient had grade 1 abdominal pain that was tolerable without dose reduction. The patient was able to maintain osimertinib combined with pemetrexed and cisplatin for 9.7 months, but she died from LM progression in June 2021. Images of chest CT and brain MRI before and after osimertinib administration are presented in Fig. 1C.
4. Case 4A 38-year-old, never-smoker female had been treated for pulmonary tuberculosis for 3 months but had not improved. By bronchoscopic biopsy, NSCLC was diagnosed, and multiple bone metastases were detected in the imaging workup. As an EGFR L868R mutation was confirmed by biopsy specimen, gefitinib was started in November 2018. Six months later, the disease had progressed, and the repeat biopsy was performed by endobronchial ultrasound-guided transbronchial needle aspiration, and an EGFR T790M mutation was not detected. The metastatic brain lesion was also identified in brain MRI and treated with GKS. Next, the patient was treated with a combination of atezolizumab, bevacizumab, paclitaxel, and carboplatin followed by atezolizumab and bevacizumab maintenance. However, in December 2019, the patient complained of nausea, vomiting, and headache, and LM was diagnosed with brain MRI and CSF cytology. Thus, osimertinib 80 mg once daily combined with pemetrexed were started. With osimertinib and systemic chemotherapy, the patient complained of myalgia and abdominal pain. However, the adverse events were well controlled with supportive care. Unfortunately, the extracranial disease was refractory, leading to additional regimen changes (docetaxel followed by gemcitabine and carboplatin). LM was uncontrolled, so additional WBRT and VP shunt insertion were necessary. Nevertheless, she died of extracranial disease progression in May 2021. Images of the chest CT and brain MRI before and after osimertinib administration are presented in Fig. 1D.
5. Survival and adverse eventsPatient characteristics and prior treatments are described in Table 1. In four reviewed cases, median follow-up duration was 37.7 months (range, 18.1 to 54.6 months) from the date of initial diagnosis of NSCLC and 12.0 months (range, 7.1 to 17.3 months) from the date of LM progression. The median time from initial diagnosis of NSCLC to LM progression was 25.5 months (range, 5.1 to 43.6 months), and the median treatment duration of osimertinib was 9.7 months (range, 6.0 to 16.8 months). On 30 August 2021, only one patient (case 2) was alive and had been ongoing treatment with osimertinib for more than 6 months combined with systemic chemotherapy. The other three patients died of LM progression. Mean OS was 49.2 (95% CI, 41.8 to 56.7) from initial NSCLC diagnosis and 14.7 months (95% CI, 10.4 to 19.0) from LM progression. Median OS from initial NSCLC diagnosis was 43.8 months (95% CI, not reached), and median OS from LM progression was not reached.
There was no ≥ 3 grade adverse event noted in the four cases, and there was one case of dose reduction due to grade 2 diarrhea (case 2). The most common adverse event was abdominal pain, which was noted in two patients (cases 3 and 4) but was well controlled with conservative care.
DiscussionWe reviewed four patients treated with combination therapy of osimertinib and cytotoxic chemotherapy for LM from EGFRm advanced NSCLC. The four patients had been diagnosed with EGFRm NSCLC and received gefitinib, a first-generation TKI, for first-line treatment. However, the disease progressed to LM without T790M mutation in repeat biopsy. These patients were treated with osimertinib combined with systemic chemotherapy for LM and extracranial lesion, respectively. Even though extracranial disease progressed in two patients, osimertinib was continuously administered until LM progression, with a median treatment duration of 11.98 months (range, 7.13 to 17.27 months). On 30 August 2021, one patient (case No. 2) was still alive and had been ongoing treatment with osimertinib plus systemic chemotherapy for more than 6 months. In four patients, there was no grade ≥ 3 adverse event, and adverse events, such as diarrhea and abdominal pain, were well controlled. In addition to the above four cases, there was another case treated with osimertinib plus systemic chemotherapy. A 75-year-old patient was never-smoker and diagnosed with EGFR mutated (exon 19 deletion) NSCLC who developed lung to lung metastasis after lobectomy. After 2 years of gefitinib, multiple brain metastases and LM were progressed in August 2020, and she was treated with WBRT and IT-MTX followed by osimertinib. However, due to progression of extracranial disease, pemetrexed/carboplatin followed by pemetrexed maintenance therapy was given, resulting in partial response. Until now, both extracranial and LM disease is well controlled with osimertinib plus chemotherapy without any serious side effects.
Because of BBB, many therapeutic agents do not reach the leptomeningeal space well. Thus, LM might be a result of pharmacological resistance rather than true resistance to TKIs. As previously mentioned, osimertinib showed superiority in BBB penetration compared to first- and second-generation EGFR TKIs [12] and improved survival in patients with LM from EGFRm NSCLC regardless of T790M status [11]. A previous study showed that osimertinib 160 mg daily was generally well tolerated [9], but there was a concern about adverse events when combined with cytotoxic chemotherapy. In AURA LM analysis, osimertinib 80 mg daily had clinical effectiveness in patients with LM from EGFR T790M-positive NSCLC [10]. Based on these points, the authors thought that osimertinib 80 mg daily combined with systemic chemotherapy would be effective and tolerable in these four patients with LM from T790M negative EGFRm NSCLC. Although there were no serious adverse events noted in this case series, one patient required dose reduction to 80 mg every other day due to grade 2 diarrhea. Thus, the performance status of the patient is thought to be very important when treatment with osimertinib plus cytotoxic chemotherapy is considered.
Attempts to overcome LM from NSCLC have been ongoing. A phase II single-arm study with osimertinib plus bevacizumab for LM from NSCLC showed a median LM OS of 12.6 months (95% CI, 9.8 to 21.2). This study enrolled a total of 14 patients (exon 19 deletion, n=7; L858R mutation, n=7) regardless of previous use of TKI (gefitinib, n=7; erlotinib, n=1; icotinib, n=1; other, n=1) [13]. Also, a new-generation EGFR TKI, ADZ3759, showed promising activity in an EGFRm mouse model of LM from NSCLC [14] and was investigated in the phase I BLOOM study [15]. However, as far as we know, there has been no trial investigating osimertinib combined with cytotoxic chemotherapy in patients with LM from EGFRm NSCLC that progressed on previous TKI treatment.
The results of this case series should be interpreted with caution. They might be affected by other treatments such as WBRT or IT-MTX for LM. Also, given the small case series, a large prospective study is needed to evaluate the efficacy of osimertinib combined with systemic chemotherapy in patients with LM from EGFRm NSCLC, especially non-T790M mutation.
In conclusion, combination therapy with osimertinib and systemic chemotherapy is a treatment option for patients with LMs and extracranial progression from T790M-negative EGFRm NSCLC resistant to previous EGFR TKI.
NotesEthical Statement This study was reviewed and approved by our institutional review board (IRB number: 2021-12-098). And this study received a written consent exemption from the IRB. Author Contributions Conceived and designed the analysis: Ahn MJ. Collected the data: Kim HR, Jo H, Kim H, Hong J, Park S, Jung HA, Lee SH, Ahn JS, Ahn MJ. Contributed data or analysis tools: Kim HR, Jo H, Kim H, Hong J, Park S, Jung HA, Lee SH, Ahn JS, Ahn MJ. Performed the analysis: Kim HR. Wrote the paper: Kim HR, Ahn MJ. Fig. 1The brain magnetic resonance imaging (MRI) and chest computed tomography (CT) imaging before and after osimertinib administration. The gray-colored arrows indicate before (left) and after (right) osimertinib administration, and the red-colored arrows point out the leptomeningeal metastasis. Brain MRI and chest CT imaging in case 1 (A), case 2 (B), case 3 (C), and case 4 (D). 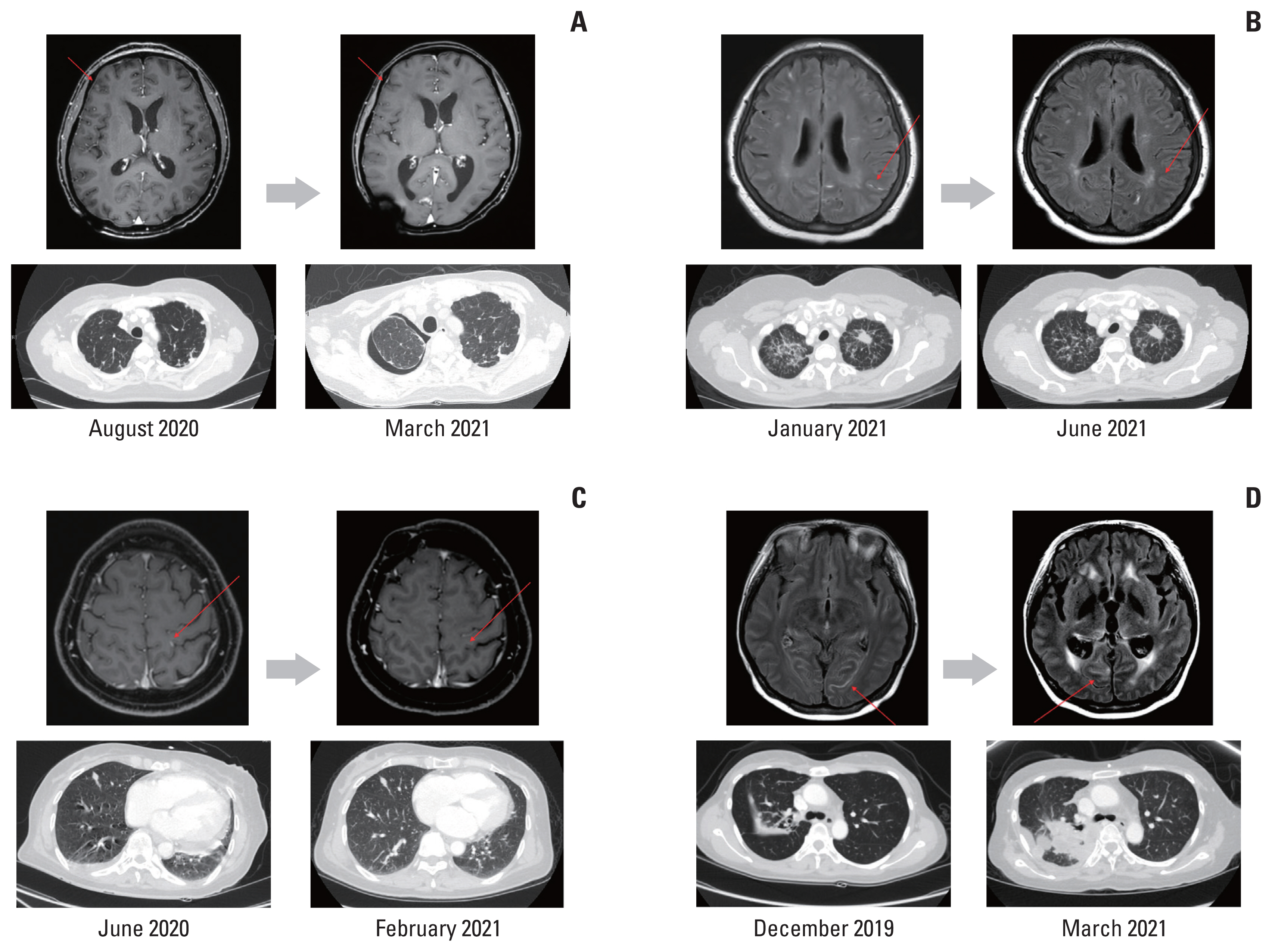
Table 1Patient characteristics, treatment, and survival DC, docetaxel and carboplatin; Del19, exon 19 deletion; EGFR, epidermal growth factor receptor; GKS, gamma-knife surgery; IT-MTX, intrathecal-methotrexate; LM, leptomeningeal metastasis; L858R, leucine-to-arginine substitution at amino acid position 858; NSCLC, non-small cell lung cancer; OS, overall survival; TC, paclitaxel and carboplatin; TKI, tyrosine kinase inhibitor; VP, ventricle-peritoneal; WBRT, whole-brain radiotherapy. References2. Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–9.
3. Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, Thunnissen E, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89:255–61.
5. Chamberlain M, Soffietti R, Raizer J, Ruda R, Brandsma D, Boogerd W, et al. Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16:1176–85.
6. Umemura S, Tsubouchi K, Yoshioka H, Hotta K, Takigawa N, Fujiwara K, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77:134–9.
7. Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8:1069–74.
8. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
9. Yang JC, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38:538–47.
10. Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15:637–48.
11. Lee J, Choi Y, Han J, Park S, Jung HA, Su JM, et al. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15:1758–66.
12. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–40.
13. Lu ZQ, Cai J, Wang X, Wei JP, Zeng ZM, Huang L, et al. Osimertinib combined with bevacizumab for leptomeningeal metastasis from EGFR-mutation non-small cell lung cancer: a phase II single-arm prospective clinical trial. Thorac Cancer. 2021;12:172–80.
|
|
|||||||||||||||||||||||||||||||||||||||||||