INTRODUCTION
The outcome of gastric cancer treatment has markedly improved as a result of better early detection and performing extensive radical operation (1,2). Indeed, the 5-year survival rate of the patients who have their gastric cancer detected early is over 90% (3), and this has greatly contributed to the improved outcome of gastric cancer treatment. Early gastric cancer is now recognized as a disease entity wia favorable prognosis after surgical treatment, and surgeons are very interested in preserving the quality of life of these patients after treatment. When considering the quality of life, minimally invasive treatments such as endoscopic mucosal resection or laparoscopic gastric surgical procedures have emerged as the frontline therapy for some of these patients (4,5). On the other hand, the prognosis of advanced gastric cancer remains far worse than that of early gastric cancer, and we actually have experienced substantial differences of recurrence and survival patterns, even in the same-staged patients. Therefore, the identification of prognostic factors, as classified by the disease stage, appears to be important for establishing appropriate therapeutic strategies. Nevertheless, there have been only a few reports on the prognostic factors according to the classification by stage.
In this study, we attempted to find the factors that are related to long survival time after curative surgery for advanced gastric cancer. We also analyzed the prognostic factors, according to the classification by stage.
MATERIALS AND METHODS
During the period from January 1993 to December 2000, 778 patients underwent surgery for advanced gastric cancer at the Department of Surgery, Korea University Hospital. Of these, 149 patients were excluded because they underwent explorative laparotomy, a bypass procedure or palliative resection. An additional 23 patients, who died from immediate postoperative complications or from other causes unrelated wigastric cancer, were excluded from the study. Curative gastric resection was performed in the remaining 606 patients, accounting for an overall resection rate of 80.8%. The surgical procedure was defined as curative when no grossly visible tumor tissue remained after resection and the resection margins were histologically normal.
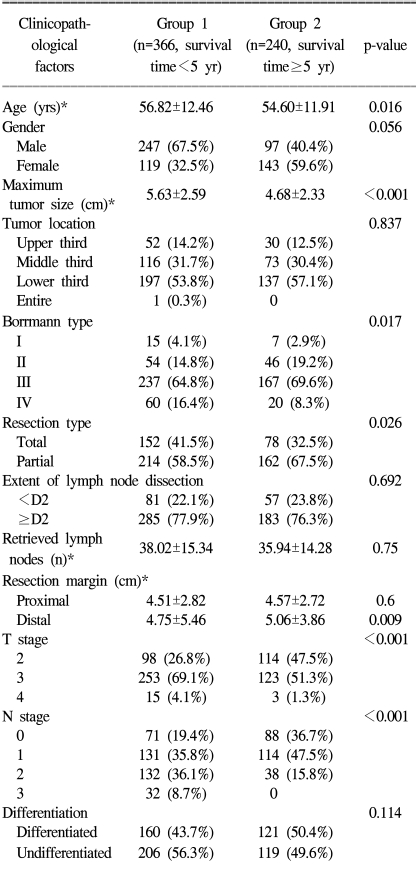
Of these 606 patients, the survival periods of 366 patients were less than 5 years (group 1), and those of the remaining 240 were over 5 years (group 2). To determine the clinicopathological factors related to the long survival of patients wiadvanced gastric cancer, we compared between the two groups for such factors as age, gender, tumor size and location, the Borrmann type, the type of resection, extent of lymph node dissection, the number of retrieved lymph nodes, the proximal and distal resection margins, the depth of tumor invasion, lymph node metastasis and the tumor histology.
We classified all these clinicopathological variables according to Japanese Classification of Gastric Carcinoma (6). Histologically, the tumors were grossly divided into the differentiated type (papillary and tubular adenocarcinoma) and the undifferentiated type (poorly differentiated adenocarcinoma, signet-ring cell carcinoma, mucinous carcinoma and miscellaneous). The stage was classified according to the 5th edition of the UICC TNM classification (7). Patient survival was evaluated using mail, telephone calls or the outpatient records.
Statistical analyses were performed with SPSS statistical software, version 13.0 for Windows (spss, Chicago, IL). Chi-squared tests and Fisher's extract test were used, whenever appropriate, to compare the categorical variables between the two groups. Logistic regression analysis was performed for the multivariate analysis. The survival curves were calculated by the Kaplan-Meier method. Differences in the survival curves were measured using log-rank tests. The independent prognostic factors were examined by Cox regression analysis. Statistical significance was defined as p-values <0.05.
RESULTS
The mean age of the 606 patients with advanced gastric cancer was 55.9 years, (range: 21 to 82 years), and there was a male predominance (male to female ratio: 1.81:1). As for the location of the primary tumor, 55.1% of the lesions were located in the distal third of the stomach, and this was followed by the middle third (31.2%) and the upper third (13.5%). According to the extent of the lymph node dissection, 138 patients (22.8%) underwent less than D2 dissection, and 468 patients (77.2%) had D2 or more lymph node dissection performed. The average number of retrieved lymph nodes was 37.2 (range: 4 to 108).
According to the UICC TNM stage classification (1997), 91 patients (15%) were in stage I b, 158 (26.1%) were in stage II, 308 (50.8%) were in stage III and 49 (8.1%) were in stage IV. The overall median survival time was 3.90±2.79 years.
1) Comparison between group 1 and group 2
The overall median survival time of the 366 patients who were included in group 1 was 2.55±1.33 years, and that of the remaining 240 patients of group 2 was 7.22±1.58 years.
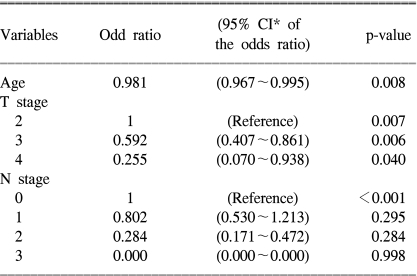
The results of univariate analysis are given in Table 1. Age, tumor size, the Borrmann type, type of resection, the distal resection margin, depth of invasion and lymph node metastasis were all significantly different between the two groups. The extent of the lymph node dissection and the number of retrieved lymph nodes were similar in the both groups. Among the significant factors that were identified by univariate analysis, multivariate analysis revealed that patient age, the depth of invasion and lymph node metastasis were the independently different factors between the two groups (Table 2).
2) Prognostic factors classified by stage
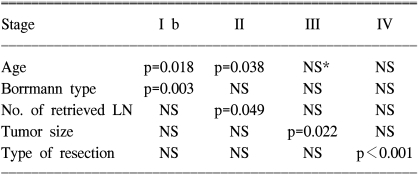
The significant prognostic factors for each stage are summarized in Table 3.
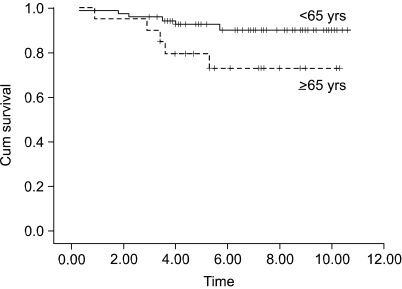
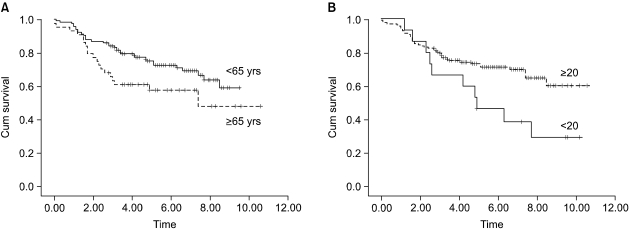
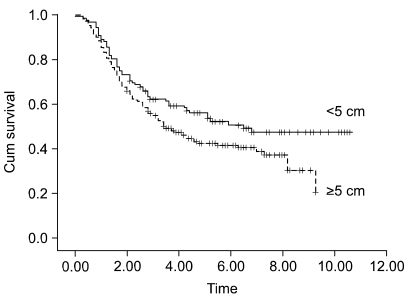
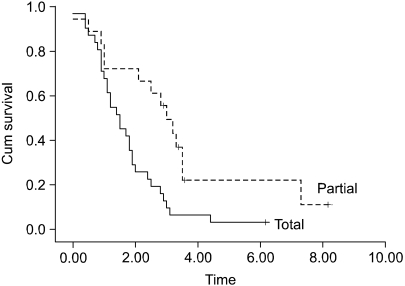
The patients at stage I b and stage II who were older than 65 years had a poor prognosis, as compared with the younger patients with the corresponding staged disease (Fig. 1, 2A). The prognosis of the patients who had more than 20 lymph nodes dissected was better than that of the patients who had less than 20 lymph nodes dissected (Fig. 2B). For the stage III disease, patients having cancer with a diameter 5 cm or less also had a better prognosis than did the patients with larger cancers (Fig. 3). For the stage IV patients, the prognosis was related to the type of resection; the prognosis after partial gastrectomy was better than that after total gastrectomy (Fig. 4).
DISCUSSION
The improved survival of gastric cancer patients is mainly due to the increased early detection of tumor (1~3). However, the results of treating advanced gastric cancer still remain dark, dismal and unpredictable; we have experienced both long survivors and sometimes a short survivor after the same treatment. In the present study, to find reasons why the prognosis is different for each patient, we investigated which clinicopathological factors could significantly affect long survival, and we found that age, depth of invasion and lymph node metastasis were the most significant prognostic factors for patients with advanced gastric cancer.
There have been controversies over the prognostic impact of patient age. Although there are some reports that age is not a prognostic factor for gastric cancer (8), older patients have generally been reported to have a poorer prognosis than do the younger patients (2, 8~11), because they have more advanced disease stage at the time of diagnosis and a lower rate of curative resection. However, patient age in our study was an independent prognostic factor for the patients who underwent curative resection. Therefore, other causes such as cardiovascular diseases, diabetics, other gerotological medical problems, defects in host immunity and malnutrition have been suggested to reflect the increased operative mortality and shortened long-term survival in older patients. Nevertheless, age alone is not likely to be a contraindication to surgery for gastric cancer patients, and careful attention should be paid to the medical and nutritional conditions of the older patients both pre- and postoperatively.
The importance of the pathological stage for the prognosis of gastric cancer is well established (8~13). The present study has also demonstrated that the pT and pN were the most significantly different variables between the long survivor group and the short survivor group (Table 1, 2).
The maximum tumor length (8) or tumor volume (14) has been reported to be an independent prognostic factor. The patients with Borrmann type IV gastric cancer have a poorer prognosis than do those patients with other types of tumor because of the delayed diagnosis and detection of advanced stage cancer, the more advanced lymph node metastasis, the more frequent peritoneal dissemination at the first operation and also the low resection rate (15,16). However, the present study failed to detect any independent prognostic value for the tumor size and Borrmann type.
The present study could not detect any significant difference in the survival according to the type of resection. Nevertheless, the worse prognosis of the patients who underwent total gastrectomy could be explained by the high incidence of gastric cancer that was found located in the upper third of the stomach, which is the cancer with more aggressive nature than the cancer at other sites (9,11,17,18).
The distal resection margin was larger in the long survivors group than in the short survivors group; however, this difference was not significant on multivariate analysis. The length of the resection margin is the grossly measured value obtained from the removed specimen. Thus, there might be a bias due to the specimens' condition, and whether or not autostaplers were used. In addition, the level of distal resection at the duodenal bulb is relatively fixed, whereas the level of proximal resection of the stomach is occasionally changeable depending on the tumor location, tumor size and the gross type. This could explain why only the distal resection margin was different and this difference failed to show significance.
The present study also failed to unravel any prognostic value for the characteristics of the long survivors group, except for the depth of invasion, lymph node metastasis and patient age: In other words, such factors as the type of resection or the extent of lymph node dissection, i.e., factors that could be chosen by the surgeon, did not appear to have any influence on the final results.
The purpose of this study was to find evidence to provide a therapeutic strategy for advanced gastric cancer patients who underwent curative resection. To achieve the goal, we analyzed the prognostic factors after stratifying them according to stage. On the analysis of the prognostic factors for stage I b to stage IV disease, which are the plausible stages of the advanced gastric cancer patients after curative resection, age was also found to be a significant prognostic factor for patients with stage I b and stage II disease, whereas age was not a factor for the patients with stage III and stage IV disease (Fig 1, 2A). Therefore, it is highly likely that once the disease progresses to the late stage, age loses its prognostic value, although it is still a significant prognostic factor for the survival of all the patients with advanced gastric cancer. The present study found that the Borrmann type was significant in stage I b. However, there was only a single case of Borrmann type IV in stage I b, therefore, its statistical significance could be a type 2 error. The number of retrieved lymph nodes was found to be a significant prognostic factor for the patients with stage II disease, and the prognosis was better after dissection of more than 20 lymph nodes than after that of less than 20 lymph nodes (Fig. 2B).
How the number of removed lymph nodes affects the survival of patients has not yet been clearly defined. Siewert et al. (8) reported that staging was reliable only when more than 15 lymph nodes were removed. They also showed a survival benefit for stage II patients, but not for all the patients, when more than 25 lymph nodes were removed, which is in agreement with our result. On the other hand, according to a report by Liu et al. (19), the survival was significantly improved when more than 15 lymph nodes were removed in patients with stage III disease. In their study, however, there were only 5 patients in stage I and II from whom more than 15 lymph nodes were removed. Furthermore, for the patients in stage III who had a survival benefit with undergoing dissection of more than 15 lymph nodes, the average number of retrieved lymph nodes was 18.3, which was much smaller than ours (37.2±15.0 for the overall 606 patients and 34.52±12.55 for the stage II patients). Thus, stage migration could have influenced their results. Although the number of retrieved lymph nodes that affects the survival benefit is thought to vary in each report according to the average number of lymph nodes that the authors removed, it is generally agreed that the number of removed lymph nodes affects the survival of stage II or stage III patients, which is the middle stage of advanced gastric cancer patients (8,19).
The rationale for the improved survival after dissection of a large number of lymph nodes for stage II or III advanced gastric cancer patients is that their disease is already considered to be systemic disease and surgical removal of micrometastatic tumor that is not usually detected on routine pathologic evaluation is necessary for achieving curative resection. The incidence of micrometastasis increases in relation to the tumor size and the depth of invasion (20), and the benefit of dissecting a large number of lymph nodes increases for those tumors. In addition, for the stage III disease of the present study, the patients whose tumor size was greater than 5 cm had a poor prognosis compared to those patients with tumor less than 5 cm in size (Fig. 3). Considering that one of the reasons for the poor prognosis of the patients with a large sized tumor is based on micrometastasis, dissection of a large number of lymph nodes would be beneficial to the survival of these patients with large tumors.
According to the type of resection, total gastrectomy showed an adverse effect on the survival of stage IV patients (Fig. 4). Among the patients with T4 or N3 disease, which are the staging components of resectable (M0) stage IV gastric cancer, we observed that the incidence of N3 was more frequent in the patients who underwent total gastrectomy than in those patients who underwent partial gastrectomy (67.7% vs 55.6%, respectively), whereas the incidence of T4 was more frequent in the patients who underwent partial gastrectomy (29.0% vs 44.4%, respectively). Although these differences were not statistically significant, lymph node metastasis has been suggested to be the major cause of the poor prognosis of the patients with cancer in the upper third of the stomach or the patients with a large sized lesion in another location who underwent total gastrectomy.
CONCLUSIONS
Patient age, the depth of invasion and lymph node metastasis were the most significant prognostic factors for advanced gastric cancer after curative resection. According to the stage, patient age had a prognostic value for the early stage of advanced gastric cancer such as stage I b or II. The number of more than 20 lymph nodes retrieved affected survival, particularly for the patients with stage II disease, and the tumor size was a significant prognostic factor for the patients with stage III disease. Thus, particular attention should be given to lymph node dissection for these patients with stage II or III disease.