AbstractPurposeWhile renal impairment is one of the first clinical manifestations of multiple myeloma (MM), declined renal function may conversely be a risk factor for cancers including MM. In this study, we investigated the relationship between chronic kidney disease and MM at a population level.
Materials and MethodsA total of 9,809,376 adults who participated in a nationwide health screening program and had no MM, cancer or end-stage renal disease at baseline were investigated for incidence of MM. The impact of estimated glomerular filtration rate (eGFR) and random urine dipstick proteinuria, and interactive associations of the two factors on the MM incidence were evaluated.
ResultsThe general incidence of MM was 4.8 per 100,000 person-years (mean follow-up of 8.3 years). Participants with eGFR < 60 mL/min/1.73 m2 (5.8% of participants) had higher MM incidence than those with eGFR ≥ 60 mL/min/1.73 m2 (adjusted hazard ratio, 1.29; 95% confidence interval, 1.17 to 1.43). When eGFR was graded into five levels, there was a significant inverse dose-response relationship between eGFR level and MM incidence at the lower eGFR levels (reference: eGFR 60–89 mL/min/1.73 m2). A dose-response relationship was also found with degree of dipstick proteinuria and incidence of MM.
IntroductionDecreased renal function is among the key features of multiple myeloma (MM). Renal failure is one of the CRAB criteria (hypercalcemia, renal failure, anemia, and bony lesions) that represents end organ damage and defines clinically significant MM or active MM [1]. Around 20%–30% of myeloma patients present with renal failure (defined as serum creatinine higher than 2.0 mg/dL) at the time of initial diagnosis [2–4]. Presence of renal failure at MM diagnosis used to be associated with poor prognosis until novel therapies such as bortezomib changed this significantly [5–7].
Urine dipstick is an inexpensive screening tool that detects albuminuria, an evidence of glomerular damage, although it has low sensitivity to Bence-Jones protein, a monoclonal immunoglobulin light chain. Bence-Jones protein is another important feature of MM, and the Durie-Salmon staging system for MM includes the level of Bence-Jones protein [8]. As monoclonal gammopathy of undetermined significance (MGUS) progresses to MM, monoclonal gammopathy-related glomerular lesions with decreased renal function and/or proteinuria may precede active MM diagnosis.
When renal impairment is present at MM diagnosis, it is often thought to be the result of MM. However, prior studies raised the possibility that chronic kidney disease (CKD) may potentially facilitate development of cancers including MM [9]. Mok et al. [10,11] showed that CKD is associated with increased cancer mortality attributed to various types of cancers including MM. While there was higher incidence of MM in patients with low estimated glomerular filtration rate (eGFR) (< 45 mL/min/1.73 m2 vs. ≥ 45 mL/min/1.73 m2) and dipstick proteinuria (1+ to 4+ vs. undetectable to trace), the total number of MM cases was only 107, limiting conclusion of a dose-response relationship and joint effects of eGFR and proteinuria.
In this study, we utilized the National Health Information Research Database (NHIRD), which is a large-scale, national database in South Korea, to investigate the relationship between renal impairment and future development of MM.
Materials and Methods1. Study cohortA retrospective cohort was created using the database of the National Health Insurance Service (NHIS). The NHIS is operated by the South Korean government, and every Korean national (50 million) is mandated to enroll. The NHIS is the lone insurer in the country and manages all the administrative processes of national health insurance. Medical service providers are largely private (~90%), and their medical services are reimbursed by the NHIS mainly on a fee-for-service payment scheme. Reimbursement requires submission of information including date of service; diagnostic codes based on the International Classification of Diseases, 10th revision (ICD-10); tests and procedures performed; and details of issued prescriptions.
The NHIS also provides free health screening services to all enrollees aged 40 and above and all employees regardless of age. In addition, NHIS provides annual screenings for workers in physical labor jobs. This screening program is mainly focused on cardiovascular risk screening and has included the following items since 2009: anthropometric measurements (blood pressure, height, weight, and waist circumference), laboratory tests (blood glucose, lipid profile, liver enzymes, serum creatinine, and dipstick proteinuria test from random urine), and a self-reported questionnaire on medical history and health behaviors (smoking, alcohol consumption, and physical activities), as previously published [12]. Blood samples were drawn after an overnight fast, and fresh, midstream urine samples were collected in the morning. Medical institutions and laboratories must be certified by the NHIS for quality control procedures to be reimbursed for their health screening programs.
2. Data sourceIn this study, we used the NHIRD of the NHIS, comprising a complete set of health information of all Korean people, linked by unique personal identifiers and includes an eligibility database containing demographic information on age, sex, place of residence, and income level; a health claims database containing medical diagnoses and treatment information (diagnosis code, tests, procedures, and prescriptions); and a health screening database. Mortality data are regularly updated by linking with vital statistics from the National Statistical Office. The NHIRD is accessible for research purposes after anonymization, with study protocol review and approval by the NHIS and has been used for various epidemiologic studies, including those investigating risk factors of various cancers. Detailed information about the NHIRD can be accessed from the NHIRD website (https://www.nhiss.nhis.or.kr), and its data structure was previously described [13–15].
3. Study populationAll people who participated in the health screening program were eligible. Among 10,505,818 subjects who participated in health screenings in 2009, subjects who were < 20 years (n=15,317); had MM (n=358), other cancers (n=157,964), or end-stage renal disease (ESRD, n=8,127) before the screening date; and had missing or abnormal test results or questionnaire responses (n=514,676) were excluded. ESRD was operationally defined as receipt of renal replacement therapy (i.e., peritoneal dialysis or hemodialysis) and/or renal transplantation with the ICD-10 diagnosis code for ESRD, as shown in a previous study [16]. The remaining 9,809,376 subjects were included in the current analysis (S1 Fig.).
4. Exposure: eGFR and dipstick proteinuriaEstimated GFR was calculated using the Modification of Diet in Renal Disease equation [17].
For the analysis, eGFR was dichotomized at 60 mL/min/1.73 m2 and was also categorized into five levels (< 30, 30–59, 60–89, 90–119, ≥ 120) based on stage of CKD [18]. Dipstick proteinuria was reported as six grades: absent, trace (±), 1+, 2+, 3+, and 4+, which correspond to urine protein levels of undetectable, 10 mg/dL, 30 mg/dL, 100 mg/dL, 300 mg/dL, and 1,000 mg/dL, respectively. As the numbers of participants with 3+ and 4+ proteinuria were small, they were combined for analyses.
5. Follow-up and study outcomesThe study population was followed from health screening date (baseline) to incidence of MM, death, or until the last follow-up date (December 31, 2017), whichever came first. Incident MM was defined as new claims for inpatient or outpatient care with diagnosis code MM (ICD-10, C90.0) linked to registration for the copayment reduction program. In Korea, all registered cancer patients receive additional discounts in copayments, and most eligible patients are expected to be captured correctly.
6. CovariatesBody mass index (BMI) was classified into underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), obese (25–29.9 kg/m2), and severely obese (≥ 30 kg/m2) according to the Asia-Pacific criteria of the World Health Organization [19]. Smoking status was classified as none, past, or current smoker, and alcohol consumption as none, mild, or heavy drinker (≥ 30 g of alcohol per day). Regular exercise was defined as performing strenuous physical activity for at least 20 minutes more than once a week. Income was classified into quartiles.
Hypertension was defined as either ICD-10 codes I10–I11, with at least one prescription of an antihypertensive agent or systolic/diastolic blood pressure ≥ 140/90 mmHg at health screening; diabetes as ICD-10 codes E10–14 with at least one prescription of antidiabetic medication or fasting glucose level ≥ 126 mg/dL; and dyslipidemia as ICD-10 code E78 with at least one prescription of lipid-lowering agent or total cholesterol ≥ 240 mg/dL.
7. Statistical analysisBaseline characteristics were described as mean±standard deviation or number and percentage, and statistical differences were tested by Student’s t-test and chi-square test. Incidence rates for MM were calculated per 100,000 person-years. The Cox proportional hazards model was used to evaluate the associations between eGFR and/or dipstick proteinuria and incidence of MM (model 1). Multivariate models accounted for (1) age and sex (model 2) and (2) age, sex, income level, smoking, physical activity, hypertension, and diabetes (model 3). Sensitivity analyses were performed with a 2-year lag period (i.e., excluding subjects with < 2 years of follow-up) to minimize the possibility of reverse causality (n=9,765,164).
The interactive effects of eGFR and dipstick proteinuria were tested using the joint combination of eGFR and proteinuria categories and were presented as incidence rates and hazard ratios based on eGFR 60–89 mL/min/1.73 m2 and no proteinuria as the reference categories. The potential effect modifications by baseline characteristics were evaluated through stratified analysis by age, sex, smoking, BMI, and interaction testing using a likelihood ratio test. Statistical analyses were carried out using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results1. Participant demographicsAmong a total of 9,809,376 participants, 569,893 (5.8%) had eGFR < 60 mL/min/1.73 m2. These participants tended to be older, female, and of lower income status. They also showed higher prevalence of dipstick proteinuria, hypertension, diabetes mellitus, and dyslipidemia and higher BMI but lower smoking and drinking rates than those who had eGFR ≥ 60 mL/min/1.73 m2 (Table 1).
2. Incidence of MM by eGFR and level of dipstick proteinuriaThe general incidence of MM in Korea was 4.8 per 100,000 person-years, which is comparable to previous reports [20,21]. The mean follow-up duration was 99.1 months (8.3 years). Comparing participants with eGFR below 60 mL/min/1.73 m2 and above 60 mL/min/1.73 m2, adjusted hazard ratio (aHR) for MM was 1.29 (95% confidence interval [CI], 1.17 to 1.43). When graded into five levels and with eGFR 60–89 as a reference group, there was a significant dose-response relationship at the lower eGFR levels: aHRs were 1.24 (95% CI, 1.11 to 1.38) for eGFR 30–59 mL/min/1.73 m2 and 1.49 (95% CI, 1.16 to 1.89) for eGFR < 30 mL/min/1.73 m2, while no decreased risk was observed in the higher eGFR groups (Table 2).
A clear dose-response relationship was also found with degree of dipstick proteinuria and incidence of MM; compared to the group with absent proteinuria, the aHRs were for MM in the trace group was 1.34 (95% CI, 1.17 to 1.67), and 5.46 (95% CI, 2.84 to 10.49) in the severe proteinuria group (Table 2). Sensitivity analyses with a 2-year lag period showed consistent results, with slight attenuation of the relative risk estimate (S2 Table).
DiscussionThis population-based cohort study involving approximately 10 million participants and 3,911 incident MM cases confirms that reduced eGFR and positive dipstick proteinuria are associated with incident MM. With the largest ever data set, we examined dose-response relationships, explored the joint effects of eGFR and dipstick proteinuria on MM risk, and tested potential interactions with baseline characteristics, i.e., age, sex, smoking status, and BMI level.
Our study showed that people with low eGFR (< 60 mL/min/1.73 m2) are 1.3 times more strongly associated with incident MM than those with higher eGFR. However, there was no linear dose-response relationship with higher or lower eGFR. A clearer dose-response relationship was observed with positive dipstick proteinuria: even the trace group had 1.34 times higher aHR for MM, while in the 3+, 4+ group, the aHR was as high as 5.46.
While the observational nature of our study excludes any definitive answers about causality, there are two possible interpretations. The first and the most plausible explanation is that renal impairment caused by monoclonal gammopathy may precede diagnosis of active MM. Most MM cases originate from progression of monoclonal gammopathy, and active MM is diagnosed when there is evidence of end organ damage as defined by the CRAB criteria [1,22]. Although renal impairment defined as serum creatinine higher than 2.0 mg/dL is included in the CRAB criteria, current guidelines only recognize renal failure caused by light-chain cast nephropathy as a myeloma-defining event [23]. However, more than 70% of renal lesions in active MM cases are associated with monoclonal protein but may be caused via several different mechanisms such as monoclonal immunoglobulin deposition disease (MIDD) or amyloid light chain (AL) amyloidosis [24]. Recognition of frequent renal impairment by monoclonal gammopathy prior to development of MM led to definition of a new entity, monoclonal gammopathy of renal significance (MGRS) [25–27]. MGRS manifests with reduced eGFR and/or proteinuria and requires treatment to prevent further damage to the kidneys. The current asymptomatic screening cohort with urine dipstick positivity without diagnosis of active MM is likely to have glomerular lesions, such as MIDD and AL amyloidosis, rather than light-chain cast nephropathy, which manifests as acute kidney injury and other symptoms of MM.
If the findings of decreased renal function represented as low eGFR and/or urine dipstick proteinuria do not lead to workups to identify monoclonal gammopathy, MGRS or active MM may remain undiagnosed. Thus, it is worth mentioning light-chain MM, which is a relatively newly described entity comprising 15% of MM. Light-chain MM produces monoclonal protein with light chains only. M-spikes on serum protein electrophoresis and serum immunofixation are frequently negative, and light-chain MM cannot be diagnosed without serum-free light chain assays. The clinical challenge of light-chain MM is that a high level of light chains is nephrotoxic and can cause renal failure. Unfortunately, light-chain MM tends to be diagnosed late, after renal failure [28]. In diagnosis of MM, comprehensive myeloma lab tests including serum-free light-chain assays based on clinical suspicion are key. Our current study convincingly shows that albuminuria and/or reduced eGFR may be early signs of MGRS or active MM.
There has been unprecedented improvement in treatment outcomes of MM during the last two decades, and this has affected the disease management. Previously, because we did not have effective myeloma therapies, watchful waiting was the standard approach for MGUS and smoldering myeloma (SMM). In 2014, the International Myeloma Working Group (IMWG) published updated MM diagnostic criteria classifying some previously defined SMM patients as active MM, for which they receive myeloma treatment. Need for SMM treatment has been the topic of hot debates in the myeloma field, and more evidence is amassing for treating SMM [29–32]. From this perspective, if there is a suspicion that plasma cell neoplasm may be causing renal impairment, complete or more aggressive workups to establish the diagnosis of MM or MGRS should be performed, as these conditions will require treatment initiation to potentially prevent end organ damage and provide more favorable outcomes.
The second possible interpretation is that renal impairment itself may play a role in development of MM. To confirm our analysis results, we performed sensitivity analysis with a 2-year lag period, and the results were similar to primary analysis. This suggests a possible causal relationship between pre-existing renal impairment and progression of monoclonal gammopathy to MM. Although it is not within the scope of our study to investigate the mechanism behind this potential causal relationship, there are several proposed mechanisms regarding how renal impairment may facilitate cancer development. Several studies have suggested that chronic inflammation and oxidative stress, which are commonly seen in the microenvironment of CKD patients, may mediate cancer development by influencing the proliferation of cells, which eventually can lead to cancer development [10,11,33–36]. This is particularly relevant in the pathogenesis of MM, because it is well known that pro-inflammatory cytokines are critical in growth of myeloma cells [37,38]. Patients on immune suppression after solid organ transplantation have higher incidences of cancers [39]. Severe renal impairment may create a weakened immune status, which increases the risk of cancer development [40]. Elucidating the mechanism of this potential relationship is critical for developing future strategies to slow the progression of MM from MGUS in patients with renal impairment. For example, it would be interesting to investigate whether interventions to slow CKD progression or interventions to reduce the level of proteinuria can inhibit MM development.
Synergistic action between low eGFR and proteinuria was not observed in our study, suggesting the independent association of each with MM. In addition, there was no interaction between eGFR or proteinuria and baseline characteristics such as age, sex, smoking, and BMI in development of MM. This provides further evidence on the robustness of our findings, regardless of participant characteristics.
Our study has several limitations. First, eGFR is an imperfect measure of renal function because serum creatinine concentration is influenced by other factors such as age, sex, muscle mass, and medications. Second, we do not have urine dipstick specific gravity data that would help correct urine concentration status. Third, we used single measurements of creatinine and dipstick proteinuria at the time of health screening and did not consider their changes over time in assessment of MM risk. Our study design does not tell us the exact date of CKD detection or the period between CKD detection and the MM incidence. Fourth, we do not have detailed information on kinds of monoclonal proteins and cytogenetics about newly diagnosed MM cases in our study. Lastly, findings from our study may not be generalizable to other populations because there are significant differences in clinical manifestation of MM depending on ethnic group.
In conclusion, our study demonstrates that reduced eGFR and positive dipstick proteinuria are associated with incident MM. These results suggest that low eGFR and albuminuria are either early signs of monoclonal gammopathy, which tends to be unrecognized, or facilitators of MM development. Clinicians should screen for monoclonal protein in patients with reduced renal function and urine dipstick proteinuria to diagnose and treat monoclonal gammopathy or MM in early stages, which will potentially reduce related organ damage and mortality.
NotesElectronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Ethical Statement Since this study involved routinely collected claims data, need for informed consent from individual participants was waived. The study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2018-06-094). Author Contributions Conceived and designed the analysis: Choi T, Shin DW, Chun S. Collected the data: Han K, Kim D. Contributed data or analysis tools: Han K, Kim D. Performed the analysis: Han K, Kim D. Wrote the paper: Choi T, Ahn W, Shin DW. Supervised the study and did critical revision of the manuscript for important intellectual content: Chun S. AcknowledgmentsThis research was funded by Samsung Medical Center, grant number OTA1604041. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
The authors thank the staff of Korean National Health Insurance Service.
Fig. 1Interactive association of estimated glomerular filtrationrate (eGFR) and dipstick proteinuria. (A) Incidence of multiple myeloma. (B) Adjusted hazard ratio of multiple myeloma. Hazard ratio was adjusted for age, sex, income level, body mass index, smoking status, regular exercise, hypertension, diabetes and dyslipidemia. Incidence rate is per 1,000 person-years. 
Fig. 2Interactions between smoking/body mass index (BMI) and estimated glomerular filtration rate (eGFR)/dipstick proteinuria. Adjusted hazard ratios for multiple myeloma incidence according to the interactions between eGFR and smoking (A), eGFR and BMI (B), urinary dipstick protein and smoking (C), and urine dipstick protein and BMI (D). p-values for interaction are 0.12 (A), 0.22 (B), 0.22 (C), and 0.28 (D). 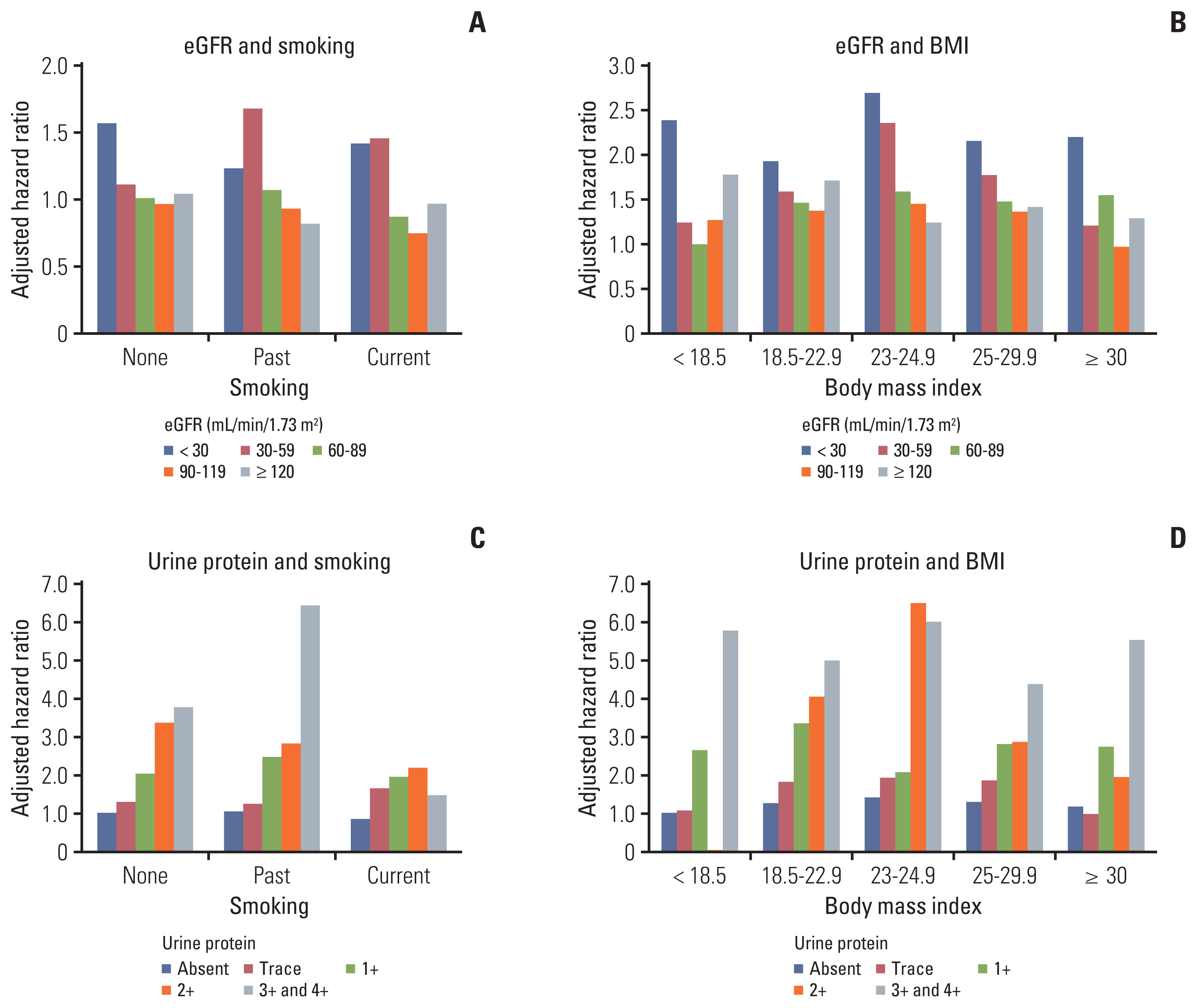
Fig. 3Interactions between age/sex and estimated glomerular filtration rate (eGFR)/dipstick proteinuria. Adjusted hazard ratios for multiple myeloma (MM) incidence according to interactions between eGFR and age (A), eGFR and sex (B), urinary dipstick protein and age (C), and urinary dipstick protein and sex (D). p-values for interaction are 0.55 (A), 0.04 (B), 0.22 (C), and 0.54 (D). 
Table 1Baseline characteristics of study participants
Table 2Incidence of multiple myeloma by estimated glomerular filtration rate and random urinary dipstick
References1. International Myeloma Working GroupCriteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
2. Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485–93.
3. Dimopoulos MA, Terpos E. Renal insufficiency and failure. Hematology Am Soc Hematol Educ Program. 2010;2010:431–6.
4. Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–84.
5. Drayson M, Begum G, Basu S, Makkuni S, Dunn J, Barth N, et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials. Blood. 2006;108:2013–9.
6. Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23:1152–7.
7. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–54.
9. Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–50.
10. Mok Y, Matsushita K, Ballew SH, Sang Y, Jung KJ, Lee S, et al. Kidney function, proteinuria, and cancer incidence: the Korean Heart Study. Am J Kidney Dis. 2017;70:512–21.
11. Mok Y, Matsushita K, Sang Y, Ballew SH, Grams M, Shin SY, et al. Association of kidney disease measures with cause-specific mortality: the Korean Heart Study. PLoS One. 2016;11:e0153429.
12. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640.
13. Seong SC, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800.
14. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15.
15. Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med. 2016;176:138.
16. Kim MK, Han K, Koh ES, Kim HS, Kwon HS, Park YM, et al. Variability in total cholesterol is associated with the risk of end-stage renal disease: a nationwide population-based study. Arterioscler Thromb Vasc Biol. 2017;37:1963–70.
17. Lamb EJ, Tomson CR, Roderick PJ. Clinical Sciences Reviews Committee of the Association for Clinical BiochemistryEstimating kidney function in adults using formulae. Ann Clin Biochem. 2005;42:321–45.
18. National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
19. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124:1045–60.
20. Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ 3rd. Incidence of multiple myeloma in Olmsted County, Minnesota: trend over 6 decades. Cancer. 2004;101:2667–74.
21. Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127:299–304.
22. Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7.
23. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
24. Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59:786–94.
25. Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711.
26. Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45–59.
27. Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292–5.
28. Sanders PW. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol. 2012;23:1777–81.
29. Hernandez JA, Martinez-Lopez J, Lahuerta JJ. Timing treatment for smoldering myeloma: is earlier better? Expert Rev Hematol. 2019;12:345–54.
30. Landgren O. Shall we treat smoldering multiple myeloma in the near future? Hematology Am Soc Hematol Educ Program. 2017;2017:194–204.
31. Lonial S, Jacobus SJ, Weiss M, Kumar S, Orlowski RZ, Kaufman JL, et al. E3A06: Randomized phase III trial of lenalidomide versus observation alone in patients with asymptomatic high-risk smoldering multiple myeloma. J Clin Oncol. 2019;37:8001.
32. Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, Lopez Corral L, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369:438–47.
34. Jorgensen L, Heuch I, Jenssen T, Jacobsen BK. Association of albuminuria and cancer incidence. J Am Soc Nephrol. 2008;19:992–8.
35. Lowrance WT, Ordonez J, Udaltsova N, Russo P, Go AS. CKD and the risk of incident cancer. J Am Soc Nephrol. 2014;25:2327–34.
36. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92.
37. Klein B, Zhang XG, Jourdan M, Boiron JM, Portier M, Lu ZY, et al. Interleukin-6 is the central tumor growth factor in vitro and in vivo in multiple myeloma. Eur Cytokine Netw. 1990;1:193–201.
38. Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–72.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||