AbstractPurposeInflammation within the tumor microenvironment has been reported to show an association with poor prognosis in breast cancer. However, the associations may differ according to breast cancer subtype. In this study, we investigated the association between inflammation-related markers and breast cancer recurrence according to patients' tumor subtypes.
Materials and MethodsThis prospective study included 240 patients who underwent surgery for management of newly diagnosed breast cancer. Levels of inflammation-related markers (interleukin [IL]-1β, IL-6, IL-8, monocyte chemoattractant protein-1 [MCP-1], leptin, and adiponectin) were measured at diagnosis, and the associations between these markers and breast cancer recurrence during a six-year follow-up period were examined using the Kaplan-Meier statistical method.
ResultsOverall, inflammation-related markers showed no association with breast cancer recurrence. However, when data were stratified by tumor subtype, higher levels of some mediators showed an association with poor prognosis among patients with particular subtypes. Compared to patients without recurrence, patients with recurrence had higher levels of circulating IL-6 (p=0.024) and IL-8 (p=0.016) only among those with HER2- tumors and had higher levels of leptin (p=0.034) only among those with estrogen receptor (ER)+/progesterone receptor (PR)+ tumors. Results of survival analyses revealed an association of high levels of IL-6 (p=0.016) and IL-8 (p=0.022) with poor recurrence-free survival in patients with HER2- tumors. In addition, higher leptin levels indicated shorter recurrence-free survival time only among patients with ER+/PR+ tumors (p=0.022).
IntroductionCorrelation of inflammation within the tumor microenvironment with tumor growth, increased invasiveness, and poor prognosis in breast cancer has been reported [1]. The tumor microenvironment is rich in inflammation-related mediators, such as immune cell-derived cytokines, chemokines, and adipocyte-derived adipokines, which are secreted by either cancer cells or tumor-associated immune cells [1]. Many studies and reviews have demonstrated an association of increased levels of circulating interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), or leptin with a poor prognosis in breast cancer patients [1,2]. It has been proposed that these mediators can stimulate proliferation and invasion of breast cancer cells directly or are involved in angiogenesis, which is essential for development and progression of breast cancer [3]. In contrast with these mediators, adiponectin, which shows negative correlation with leptin expression, is anti-angiogenic and anti-proliferative, and some studies have reported reduced adiponectin levels in breast cancer patients [2]. Therefore, these inflammation-related markers may be useful in prediction of prognosis and identification of cases with a high risk for metastasis [1,4].
Breast cancer is a complex and heterogeneous disease, and its prognosis may depend on characteristics of tumor and host [5]. Therefore, the roles of these inflammation-related markers in mediation of tumor growth and metastasis could be influenced by distinct subtypes that have been identified on the basis of gene or protein expression in tumor tissue [4]. Different breast cancer subtypes may produce distinct inflammatory mediators, which may affect their distinct tumor progression pathways [6,7]. The aim of the current study was to investigate the association between breast cancer recurrence and inflammation-related markers, including IL-1β, IL-6, IL-8, MCP-1, leptin, and adiponectin, according to patients' tumor subtype.
Materials and Methods1. Study participants and follow-upThis was a prospective study of newly diagnosed breast cancer patients who underwent surgery at the National Cancer Center Hospital in Korea between July 2007 and September 2008, with follow-up through January 2013. Among 441 breast cancer patients, 26 patients did not agree to participate in the study and 105 patients refused to provide their blood for the study. Among 310 women, we excluded patients with a previous history of cancer (n=14) and those with stage 0 or IV cancer (n=56). The remaining 240 patients were included in the final analysis. Those patients were followed up for six years in order to identify cases of breast cancer recurrence. Breast cancer recurrence included local (n=8) or distant metastasis (n=23), and 31 recurrent patients were identified. Eleven recurrent patients died of breast cancer. Each participant provided written informed consent, and the procedure was approved by the Institutional Review Board of the National Cancer Center (IRB protocol number NCCNCS 07-083).
2. Data collectionParticipants were interviewed in person by a trained researcher using a structured questionnaire. Data collected in baseline evaluations included demographic characteristics, personal and family medical history, alcohol consumption, smoking history, hormone replacement therapy, and age at menarche or menopause. Blood samples were collected at diagnosis of breast cancer and stored at -80℃ until analysis. The plasma concentrations of IL-1β, IL-6, IL-8, MCP-1, leptin, and adiponectin were quantified using the human Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions; absorbance was read on a plate reader (Biotech Instruments Inc., Winooski, VT).
3. Evaluation of breast cancer clinicopathological factorsWe evaluated conventional clinicopathological factors, including adjuvant treatment modalities (hormone therapy and anti-HER2-therapy), tumor subtype, and Ki-67 index. Immunohistochemistry (IHC) of four different biological factors (estrogen receptor [ER], SP1, Ventana, Tucson, AZ; progesterone receptor [PR], 1E2, Ventana; HER2, polyclonal, Dako, Glostrup, Denmark; Ki-67, MIB-1, Dako) was performed using paraffin-embedded breast tumor sections according to reported recommendations for tumor marker prognostic studies (REMARK) [8]. ER and PR positivity was defined using a cut-off value of 10% or more of positively stained nuclei [9]. HER2 was scored as 0-3+ according to the method recommended for the Dako Hercep Test [10]. The HER2 status of each patient was defined as follows: HER2-positive (HER2+) if the IHC score was 3+, HER2-negative (HER2-) if the IHC score was 0 or 1+, or indeterminate if the IHC score was 2+. For indeterminate patient samples, further analysis was performed using fluorescence in situ hybridization (FISH); if FISH was not available, the patients were considered HER2+-unknown [11].
For assessment of Ki-67 in breast cancer, cells stained for Ki-67 were counted and expressed as a percentage. Ki-67 index of less than 15% was assessed as low expression [12]. The pathological tumor stage was assessed according to the criteria established by the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual [13]. The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis [14].
4. Statistical analysesAll statistical analyses were performed using SAS ver. 9.1 (SAS Institute Inc., Cary, NC). A two-sided p-value of less than 0.05 was regarded as statistically significant.
For evaluation of differences in patients' clinocopatholgical characteristics (cancer stage, tumor subtype, tumor size, lymph node metastasis, Ki-67 index, histologic grade, and treatments) according to the levels of inflammation-related markers (IL-1β, IL-6, IL-8, MCP-1, leptin, and adiponectin), chi-square test and Kruskal Wallis test were used for categorical variables and continuous variables, respectively. Patients were divided into two groups (high/low) based on their median values of inflammation-related markers. In addition, patient characteristics (age, body mass index [BMI], smoking, alcohol intake, menopausal status, and clinicopathologic characteristics) were compared in relation to recurrence status using the Kaplan-Meier statistical method for estimation of recurrence-free survival, and the log-rank test for comparison of differences in recurrence-free survival. Recurrence-free survival was calculated from the day of sampling until breast cancer recurrence, death, or the end of the study period. Local and distant relapses were considered as recurrences. To investigate the association between the levels of inflammation-related markers and recurrence of breast cancer, the median levels of inflammation-related markers were compared according to patients' recurrence status; significant differences were identified using the median test. In addition, the Kaplan-Meier statistical method and the log-rank test were used for comparison of patients' recurrence-free survival rates according to the levels of inflammation-related markers. The Cox proportional hazards regression model was used to control for multiple factors simultaneously, and for estimation of the adjusted hazard ratios and the 95% confidence intervals. The following covariates were considered as potential confounders:
age, stage of disease, menopausal status, tumor subtypes, and tamoxifen treatment.
For all analyses, subgroup analyses were performed based on tumor subtype. Patients were divided into subgroups as follows: 1) four tumor subtypes: luminal A (ER+ and/or PR+, HER2-, and Ki-67 index<15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), and triple-negative (ER-, PR-, and HER2-); 2) ER/PR status: ER+/PR+ and ER-/PR-; 3) HER2 status: HER2+ and HER2-.
ResultsThe median follow-up period was 57.9 months (interquartile range, 54.4 to 60.9 months) from the date of the initial breast cancer surgery. Based on tumor subtype, patients were divided into subgroups, as follows: in regard to the four tumor subtypes, 40.7% of patients had luminal A, 28.1% of patients had luminal B, 10.0% of patients had HER2+, and 21.3% of patients had triple negative tumors; in regard to ER/PR status, 71.3% of patients had ER+/PR+ tumors and 28.7% of patients had ER-/PR- tumors; in regard to HER2 status, 18.1% of patients had HER2+ tumors and 81.9% of patients had HER2- tumors.
Table 1 shows the different clinicopathological characteristics of patients according to the levels of inflammation-related markers (IL-1β, IL-6, IL-8, MCP-1, leptin, and adiponectin). No differences were observed with respect to breast cancer recurrence for all inflammation-related markers tested. However, differences in some clinicopathlogical prognostic factors were observed according to the levels of inflammation-related markers; IL-1β levels differed by tumor subtype (p=0.045); high IL-6 levels showed an association with advanced cancer stage (p=0.038) and lymph node metastasis (p=0.007); high MCP-1 levels showed an association with advanced cancer stage (p=0.025) and HER2+ tumors (p=0.016); high leptin levels showed an association with HER2+ tumors (p=0.016); high adiponectin levels showed an association with smaller tumor size (p=0.029).
Table 2 shows characteristics of patients according to recurrence status. No differences with respect to age, BMI, smoking status, alcohol intake, menopausal status, histologic grade, and treatments were observed between patients whose cancer recurred and those without recurrence. Patients who were in an advanced cancer stage (p=0.005), T stage (p=0.002) and N stage (p=0.002), and had higher Ki-67 index (p=0.013) had poor recurrence-free survival.
We examined the median plasma levels of inflammation-related markers (IL-1β, IL-6, IL-8, MCP-1, leptin, and adiponectin) according to patients' recurrence status, stratified by their tumor subtype. Overall, inflammation-related markers did not differ according to patients' recurrence status. However, different plasma levels of IL-6, IL-8, and leptin were observed according to patients' recurrence status only among patients with certain tumor subtypes (Table 3). Analysis of IL-6 levels according to patients' recurrence status showed higher levels of IL-6 in patients with triple negative (p=0.024) or HER2- (p=0.024) breast cancer with recurrence than in those without recurrence. Regarding IL-8, HER2- breast cancer patients with recurrence had higher IL-8 levels than those without recurrence (p=0.016). Regarding leptin, ER+/PR+ patients with recurrence had higher leptin levels than those without recurrence (p=0.034). However, the levels of IL-1β, MCP-1, and adiponectin did not differ according to patients' recurrence status in all subtypes tested (data not shown).
Finally, we compared recurrence-free survival according to the levels of inflammation-related markers. Overall, inflammation-related markers showed no association with recurrence-free survival. However, when data were stratified by breast cancer subtypes, significant associations were observed. Among patients with HER2- tumors, high levels of IL-6 (p=0.016) and IL-8 (p=0.022) showed an association with poor recurrence-free survival (Figs. 1 and 2). Among patients with ER+/PR+ tumors, high leptin levels showed an association with shorter recurrence-free survival time (p=0.022) (Fig. 3). We also performed Cox proportional hazards regression analyses, adjusting for possible confounders, however, none of the associations were statistically significant (data not shown).
DiscussionPrevious evidence has indicated that inflammation within the tumor microenvironment may play an important role in breast cancer progression [15]. Most previous studies have reported an association of high levels of circulating proinflammatory cytokines with poor prognosis in breast cancer [1,3]. These proinflammatory cytokines may stimulate tumor cell motility and invasion for enhancement of metastasis of tumor cells. These cytokines are also chemoattracting and mitogetic for promotion of tumor growth [2]. In the current study, we investigated the prognostic role of certain cytokines in breast cancer progression. We observed an association of levels of IL-6, IL-8, or leptin with breast cancer recurrence; these associations differed according to tumor subtype. Some studies have found that cytokines can enhance, inhibit, or have no effect on cell proliferation and differentiation depending on the cell type examined, implying that the role of cytokines in mediation of tumor growth could be affected by tumor subtype [16]. Each tumor subtype may communicate differently with the immune system and produce a distinct cytokine profile [6,7], which may have different effects on tumor progression.
High levels of IL-6 or IL-8 were known to be associated with breast cancer recurrence [16-18]. However, the current results implied that the role of these cytokines in breast cancer recurrence may differ according to HER2 status; levels of IL-6 and IL-8 showed a positive association with breast cancer recurrence only among patients with HER2- tumors. HER2 is a transmembrane tyrosine kinase receptor that mediates growth, differentiation, and survival of cells; overexpression of HER2 at the cell membrane may lead to activation of multiple signaling complexes [5]. Some studies have reported different immune-mediated mechanisms according to patients' HER2 status and implied that abnormal expression of HER2 in breast tissue may affect the complex interaction between cancer and the immune system [19,20]. In a recent experimental study using the MMTV-NeuT mouse model, Ciampricotti et al. [20] found that HER2-driven breast tumorigenesis and metastasis formation is independent of the adaptive immune system. This finding might imply that the composition of the cytokine profile of the inflammatory tumor microenvironment is not associated with prognosis of HER2+ breast cancer; thus, the role of mediators in cancer progression was observed only among patients with HER2- tumors. However, more evidence is needed in order to elucidate the underlying mechanism of the differential association according to HER2 status.
We also observed a positive association between leptin and breast cancer recurrence, only among patients with hormone receptor positive tumors. Recent studies have indicated an association of obesity with breast cancer progression. Leptin, a hormone whose expression is elevated in overweight and obese people, may play a role in cell growth, motility, and invasiveness in cancer cells [21]. Evidence has indicated that leptin and estrogen might cooperate in maintaining estrogen-dependent breast cancer growth [21]. Leptin can increase aromatase activity, promote estrogen production, and, thus, stimulate progression of ER+ breast cancer [22,23]. Estradiol has also been reported to induce expression of leptin and leptin receptor in MCF-7 breast cancer cells [24]. Growth of estrogen-dependent breast cancer is caused mainly by ER signaling that could be activated by leptin signaling [21], which may explain the positive association between leptin and breast cancer recurrence among ER+ breast cancer patients in the current study.
In addition to tumor subtype, other tumor characteristics may influence the role of cytokines in breast cancer progression. IL-6-mediated effects on breast cancer progression have been suggested to differ according to the stage of the disease; correlation of IL-6 expression in early breast carcinoma with good prognosis has been reported [25], while IL-6 expression in advanced disease may contribute to breast cancer progression [17]. In the current study, the levels of IL-6 showed an association with cancer stage and lymph node metastasis. Considering the association between these clinicopathological features and breast cancer recurrence, the role of IL-6 in breast cancer recurrence should be investigated further. However, in this study, IL-8 levels did not show correlation with any clinicopathological prognostic factor and could be a better independent prognostic factor. In addition, hormone receptor or HER2-targeted agents, such as tamoxifen or trastuzumab, may influence the roles of cytokines and differentially affect cytokine secretion [4].
The association between circulating inflammation-related markers and breast cancer recurrence was examined prospectively in the current study; however, we must note several limitations in interpreting these results. This study suffers from a lack of statistical power because it included only 240 patients, of whom only 31 patients (12.9% of patients) experienced disease recurrence in the relatively short follow-up period. In addition, given the multiple comparisons, the significant results for IL-6, IL-8, or leptin could be a chance finding. Conduct of additional larger studies will be required in order to validate the findings from the current study.
ConclusionThe present study implied that certain cytokines, such as IL-6, IL-8, and leptin may be associated with the prognosis of breast cancer among patients with particular tumor subtypes. We cautiously speculate that tumor subtype-specific approaches that regulate cytokine levels could be a therapeutic option for reducing the risk of recurrence and improving the prognosis of breast cancer. However, conduct of larger studies will be required in order to determine the precise roles of these cytokines and their interactions with other factors in breast cancer progression.
AcknowledgmentsThis study was funded by the Korean Science and Engineering Foundation (R01-2007-000-11293-0), and supported in part by an NCC Grant (1210530-1).
References1. Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. PMID: 20545607
2. Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. PMID: 23121183
3. Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. PMID: 16931107
4. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. PMID: 18846100
5. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. PMID: 19574486
6. Gonzalez RM, Daly DS, Tan R, Marks JR, Zangar RC. Plasma biomarker profiles differ depending on breast cancer subtype but RANTES is consistently increased. Cancer Epidemiol Biomarkers Prev. 2011;20:1543–1551. PMID: 21586622
7. Levano KS, Jung EH, Kenny PA. Breast cancer subtypes express distinct receptor repertoires for tumor-associated macrophage derived cytokines. Biochem Biophys Res Commun. 2011;411:107–110. PMID: 21712030
8. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. PMID: 16172462
9. Regitnig P, Reiner A, Dinges HP, Hofler G, Muller-Holzner E, Lax SF, et al. Quality assurance for detection of estrogen and progesterone receptors by immunohistochemistry in Austrian pathology laboratories. Virchows Arch. 2002;441:328–334. PMID: 12404057
10. Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999;17:1983–1987. PMID: 10561248
11. Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20PMID: 18307763
12. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. PMID: 20152769
13. In : Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th edNew York: Springer; 2010. p. 347–376.
14. Henson DE, Ries L, Freedman LS, Carriaga M. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer The basis for a prognostic index. Cancer. 1991;68:2142–2149. PMID: 1913453
15. Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. PMID: 19470918
16. Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. PMID: 12494472
17. Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–1726. PMID: 12771987
18. Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–7162. PMID: 15534087
19. Muraro E, Martorelli D, Turchet E, Miolo G, Scalone S, Comaro E, et al. A different immunologic profile characterizes patients with HER-2-overexpressing and HER-2-negative locally advanced breast cancer: implications for immune-based therapies. Breast Cancer Res. 2011;13:R117PMID: 22112244
20. Ciampricotti M, Vrijland K, Hau CS, Pemovska T, Doornebal CW, Speksnijder EN, et al. Development of metastatic HER2(+) breast cancer is independent of the adaptive immune system. J Pathol. 2011;224:56–66. PMID: 21480230
21. Barone I, Catalano S, Gelsomino L, Marsico S, Giordano C, Panza S, et al. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012;72:1416–1427. PMID: 22282662
22. Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. PMID: 12734209
23. Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–19915. PMID: 14985328
24. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. PMID: 16533767
25. Karczewska A, Nawrocki S, Breborowicz D, Filas V, Mackiewicz A. Expression of interleukin-6, interleukin-6 receptor, and glycoprotein 130 correlates with good prognoses for patients with breast carcinoma. Cancer. 2000;88:2061–2071. PMID: 10813718
Fig. 1Effect of interleukin 6 (IL-6) on breast cancer recurrence-free survival according to HER2 status: (A) HER2 positive and (B) HER2 negative. 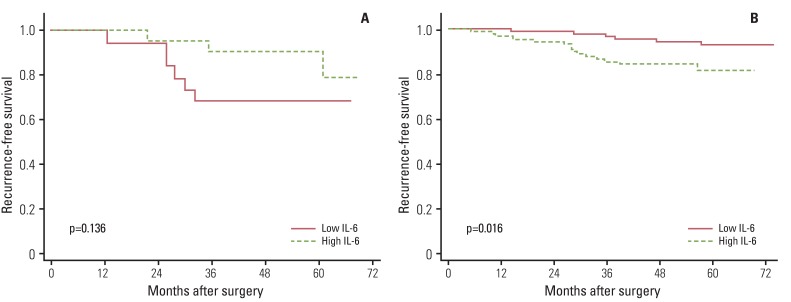
Fig. 2Effect of interleukin 8 (IL-8) on breast cancer recurrence-free survival according to HER2 status: (A) HER2 positive and (B) HER2 negative. 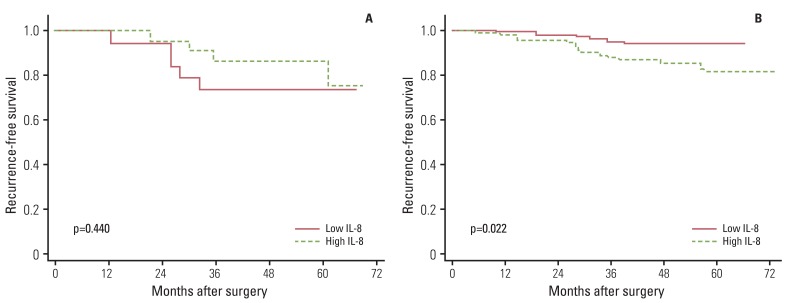
Fig. 3Effect of leptin on breast cancer recurrence-free survival according to estrogen receptor (ER)/progesterone receptor (PR) status: (A) ER/PR positive and (B) ER/PR negative. 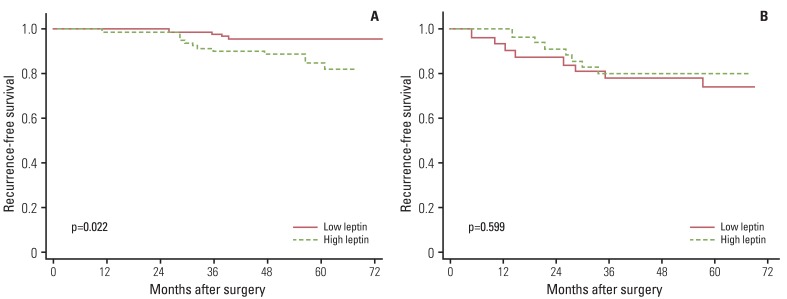
Table 1Patients' clinicopathological characteristics according to the levels of inflammation-related markersa)
IL, interleukin; MCP-1, monocyte chemoattractant protein-1; ER, estrogen receptor; PR, progesterone receptor. a)Data are presented as number (%), b)Luminal A (ER+ and/or PR+, HER2- and Ki-67 index<15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-), c)The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis. *p<0.05. Table 2Patients' characteristics according to recurrence status
ER, estrogen receptor; PR, progesterone receptor. a)Recurrence-free survival rate, b)Kaplan-Meier statistical method, compared using the log-rank test, c)The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis, d)Luminal A (ER+ and/or PR+, HER2-, and Ki-67 index<15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-), e)The effect of tamoxifen on breast cancer recurrence was compared among patients with hormone receptor positive breast cancer, f)The effect of anti-HER2 therapy, including trastuzumab (Herceptin) and lapatinb (Tykerb), on breast cancer recurrence was compared among patients with HER2+ breast cancer. Table 3Median plasma levels of inflammation-related markers according to patients' recurrence status, stratified by tumor subtype
Values are presented as number or median (interquartile range). IL, interleukin; ER, estrogen receptor; PR, progesterone receptor. a)The median test was used for identification of significant differences, b)Luminal A (ER+ and/or PR+, HER2-, and Ki-67 index <15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-). |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||