AbstractPurposeThis study aims to evaluate the prognosis of pathologically node-positive bladder cancer after neoadjuvant chemotherapy, the role of adjuvant chemotherapy in these patients, and the value of preoperative clinical evaluation for lymph node metastases.
Materials and MethodsPatients who received neoadjuvant chemotherapy followed by partial/radical cystectomy and had pathologically confirmed lymph node metastases between January 2007 and December 2019 were identified and analyzed.
ResultsA total of 53 patients were included in the study. The median age was 61 years (range, 34 to 81 years) with males comprising 86.8%. Among the 52 patients with post-neoadjuvant/pre-operative computed tomography results, only 33 patients (63.5%) were considered positive for lymph node metastasis. Sixteen patients (30.2%) received adjuvant chemotherapy (AC group), and 37 patients did not (no AC group). With the median follow-up duration of 67.7 months, the median recurrence-free survival (RFS) and the median overall survival (OS) was 8.5 months and 16.2 months, respectively. The 2-year RFS and OS rates were 23.3% and 34.6%, respectively. RFS and OS did not differ between the AC group and no AC group (median RFS, 8.8 months vs. 6.8 months, p=0.772; median OS, 16.1 months vs. 16.3 months, p=0.479). Thirty-eight patients (71.7%) experienced recurrence. Distant metastases were the dominant pattern of failure in both the AC group (91.7%) and no AC group (76.9%).
IntroductionPlatinum-based neoadjuvant chemotherapy improves survival in patients with muscle-invasive bladder cancer [1]. The prognostic value of the pathological response to neoadjuvant chemotherapy is also well appreciated [1]. Neoadjuvant chemotherapy induces downstaging of the tumor in a significant proportion of patients. However, despite neoadjuvant chemotherapy, about 33% of patients are found to have extravesical diseases (ypT3–4), and about 16%–20% have lymph node metastases (ypN+) on radical cystectomy specimens [2,3].
Among poor responders to neoadjuvant chemotherapy, patients with ypN+ are expected to have much worse prognoses [4], with reported 5-year survival rates of approximately 20% [3]. However, few studies dedicated to patients with ypN+ disease have been reported and little is known regarding the clinical features, and particularly, the role of preoperative restaging computed tomography (CT) and pattern of relapse in these patients.
Although cisplatin-based adjuvant chemotherapy can improve recurrence-free survival (RFS) and has been suggested to improve overall survival (OS) for chemotherapy-naïve patients with the extravesical or node-positive disease after radical cystectomy [5], the role of adjuvant chemotherapy is unclear in patients with significant residual disease (extravesical or node-positive disease) after neoadjuvant chemotherapy. A limited number of retrospective studies have addressed the effectiveness of adjuvant chemotherapy in these patients and showed contradictory results [2,6–8]. Therefore, current guidelines do not recommend adjuvant chemotherapy for these patients, based on the lack of evidence.
In this study, we assessed the prognosis of patients with ypN+ bladder cancer after neoadjuvant chemotherapy, along with the role of adjuvant chemotherapy, the value of preoperative clinical evaluation for lymph node metastases, and potential prognostic factors in these patients.
Materials and Methods1. PatientsFrom January 2007 to December 2019, patients with muscle-invasive bladder cancer who had received neoadjuvant chemotherapy followed by partial or radical cystectomy and had pathologically confirmed lymph node metastases on the surgical specimen were retrospectively identified and included in the study. Patients with lymph node metastases beyond common iliac nodes at diagnosis were also included if they were considered negative for distant lymph node metastases at the time of surgery. Patients who had gross residual disease after surgery were excluded.
2. Neoadjuvant chemotherapy, surgery, and adjuvant chemotherapyNeoadjuvant chemotherapy consisted primarily of cisplatin-based regimens, such as gemcitabine/cisplatin (GC; gemcitabine 1,000 mg/m2 on days 1 and 8 and cisplatin 70 mg/m2 on day 1 every 3 weeks), or high-dose-intensity methotrexate, vinblastine, adriamycin, and cisplatin (HD-MVAC; methotrexate 30 mg/m2 on day 1, vinblastine 3 mg/m2, doxorubicin 30 mg/m2, and cisplatin 70 mg/m2 on day 2, and granulocyte-colony stimulating factor [G-CSF] 240 μg/m2 from days 4–10 or long-acting G-CSF [pegfilgrastim] 6 mg on day 3 every 2 weeks). For patients who had limited renal function (glomerular filtration rate 45–60 mL/min), gemcitabine with split-dose cisplatin (GC split, gemcitabine 1,000 mg/m2 on days 1 and 8 and cisplatin 35 mg/m2 on day 1 and 2 or day 1 and 8 every 3 weeks). Patients without clinical node metastases (cN0) at baseline received four cycles of chemotherapy, whereas those with cN1 received six cycles of chemotherapy as long as there was no evidence of disease progression on response assessment and adverse events were tolerable.
Pelvic lymph node dissection (PLND) was performed per a standardized template. All patients who underwent radical cystectomy received standard PLND, which included the obturator, internal, and common iliac lymph nodes. Extended PLND, including lymph nodes extending above the common iliac bifurcation [9] was given at the discretion of the surgeon.
The decision to give adjuvant chemotherapy was based on patients’ preference and physicians’ recommendations according to the performance and tolerability of neoadjuvant chemotherapy. For adjuvant chemotherapy, the GC regimen was given for patients who received HD-MVAC as neoadjuvant chemotherapy, and HD-MVAC or MVAC regimen was given for those who received GC as neoadjuvant chemotherapy.
3. AssessmentClinical staging was assessed at two-time points: (1) at diagnosis, which was described as cTNM stage, and (2) after completion of neoadjuvant chemotherapy and before surgery, which was described as ycTNM stage. The clinical nodal stage was assessed by a dedicated independent genitourinary radiologist (K.J.P.) based on the size (≥ 8 mm in short-axis diameter), shape, and internal architecture of the lymph nodes such as necrosis or preservation of normal fatty hilum [10]. The pathological response was determined by the findings on cystectomy and PLND specimen, which were evaluated according to our institutional standard protocol. Patients were staged by the eighth edition of the American Joint Committee on Cancer staging system [11].
4. Statistical analysisBaseline characteristics were assessed by a descriptive method. RFS was defined as the time between the date of surgery and the date of radiologically confirmed tumor recurrence or death, whichever occurred first. OS was defined as the time from the date of surgery to the date of death of any cause. Survival outcomes were estimated using the Kaplan-Meier method and compared by a log-rank test. Univariable and multivariable prognostic factor analyses were performed using the Cox proportional hazards model. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using R ver. 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results1. PatientsDuring the study period, 61 patients were identified to receive neoadjuvant chemotherapy followed by partial or radical cystectomy and had ypN+ disease. Among those, eight were excluded due to the presence of grossly residual disease after surgery. A total of 53 patients were analyzed. The baseline characteristics of the study population are listed in Table 1. The median age was 61 years (range, 34 to 81 years) with males comprising 86.8% of the patients, without significant differences between patients who received adjuvant chemotherapy (AC group, n=16) and those who did not (no AC group, n=37). In the non-AC group, reasons for not receiving adjuvant chemotherapy were identifiable in 28 patients. Among those, decision after discussions on the risk-benefit of the adjuvant chemotherapy was the most common cases (12 patients), followed by inadequate performance status of the patient for chemotherapy (7 patients).
The AC group included a higher proportion of patients with ypIVA disease, whereas the no AC group tended to include a higher proportion of patients with ypT3+ disease. Forty-nine out of 53 patients (92.5%) received standard or extended PLND. Postoperative 30- and 60-day discharge rate, post-discharge 60-day readmission rate, and cisplatin eligibility after surgery did not differ between groups (S1 Table).
2. Preoperative evaluation for node positivityThe sensitivity of preoperative clinical staging (ycTNM) for the prediction of pathological staging (ypTNM) was assessed in 52 patients with post-neoadjuvant/pre-operative CT results. Thirty-three patients were identified to have ycN+ disease at preoperative/post-neoadjuvant chemotherapy evaluation, which resulted in an overall sensitivity of preoperative clinical staging of 63.5%. The number of ycN+ lymph nodes was not correlated with the number of ypN+ lymph nodes (R=0.046, p=0.748 by Pearson’s correlation). The number of patients whose ycN category and ypN category were concordant was 13 (25.0%).
3. Survival outcomes and effectiveness of adjuvant chemotherapyWith the median follow-up duration of 67.7 months (95% confidence interval [CI], 36.0 to 84.4), the median RFS was 8.5 months (95% CI, 6.0 to 13.6) and median OS was 16.2 months (95% CI, 9.9 to 19.0) in the entire study population. RFS rate at 1, 2, 5 years were 38.2%, 23.3%, and 18.2%, respectively, and the OS rate were 63.1%, 34.6%, and 25.2%, respectively. No significant differences in the RFS and OS were noted between the AC group and no AC group (Fig. 1); median RFS was 8.8 months (95% CI, 6.0 to 18.1) in the AC group vs. 6.8 months (95% CI, 4.2 to 15.1) in the no AC group (p=0.772). Median OS was 16.1 (95% CI, 7.7 to 31.6) in the AC group vs. 16.3 months (95% CI, 9.9 to 19.0) in the no AC group (p=0.479). Similarly, no significant differences in the RFS and the OS were observed in the subgroup of patients with ycN0, ypN+M0 diseases, and in patients who achieved complete resection (R0 resection) (S2 Fig.).
Regimens, cycles, and reasons for discontinuation of adjuvant chemotherapy are summarised in Table 2. The most commonly used regimen was MVAC (12 out of 16 patients, 75.0%). However, only five out of 16 patients (31.2%) completed preplanned adjuvant chemotherapy cycles. Reasons for discontinuation were disease progression (n=5, 31.2%), adverse events (n=5, 31.2%), and patient’s refusal (n=1, 6.2%). Five out of 16 patients (31.5%) received only one cycle of adjuvant chemotherapy.
4. Recurrence patternOverall, 38 out of 53 patients (71.7%) experienced recurrence during follow-up. Distant metastases were the dominant pattern of failure in both the AC group (91.7%) and no AC group (76.9%). The addition of adjuvant chemotherapy did not result in decreased incidence of distant metastases (p=0.522) (Table 3).
5. Prognostic factor analysisUnivariable and multivariable analyses for RFS and OS were performed (S3 and S4 Tables). Multivariable analysis showed that the number of positive lymph nodes (≥ 3) was associated with poor RFS (hazard ratio [HR], 2.29 [95% CI, 1.11 to 4.70], p=0.024), and the number of harvested lymph nodes at surgery (≥ 20) was associated with favorable RFS. (HR, 0.40 [95% CI, 0.18 to 0.88]; p=0.022). Old age (≥ 70 years) was associated with poor OS in the univariable analysis only. Adjuvant chemotherapy was not associated with RFS or OS, both in univariable and multivariable analyses.
DiscussionIn this study, the survival outcomes of patients with ypN+ disease after neoadjuvant chemotherapy were poor with a median RFS of 8.5 months and a median OS of 16.2 months. RFS at 2 years was 23%, implying that 77% of the patients experience recurrence or death within the first 2 years after surgery. Although limited in number, previous retrospective studies also reported poor prognoses in patients with pathological node-positive bladder cancer after neoadjuvant chemotherapy with a median OS of 13–30 months [2,4,6,8,12]. However, about 25% of our study population achieved long-term survival at 5 years, consistent with prior studies [3,6]. These unsatisfactory outcomes and yet the presence of occasional long-term survivors, therefore, bring additional questions, such as the role of additional treatments including adjuvant chemotherapy, extended lymph node dissection, or adjuvant radiotherapy.
The addition of adjuvant chemotherapy did not yield a significant survival benefit in our study. The role of adjuvant treatment in those who remained node-positive after neoadjuvant chemotherapy is rather unclear. Previous studies often included patients with advanced ypT3+ disease along with ypN+ patients [2,6,8]. The feasibility of adjuvant chemotherapy is also an important factor to consider. One-third of the patients in the AC group of our study experienced recurrence during the course of adjuvant treatment. Moreover, the remaining patients were often intolerable to treatment [13]. Additionally, adjuvant chemotherapy may not be effective in patients with pN+ disease, irrespective of neoadjuvant chemotherapy. A subgroup analysis of the EORTC 30994 study showed adjuvant chemotherapy effect varied according to pN category (p=0.026) and only patients with pN− disease had OS benefit from adjuvant chemotherapy. Furthermore, considering that our study population had advanced disease despite neoadjuvant chemotherapy, indicating relative unresponsiveness to chemotherapy, they were not likely to benefit from additional chemotherapy. Although the role of adjuvant chemotherapy appears to be minimal in this study, further studies are needed in settings where meaningful clinical responses to neoadjuvant chemotherapy were observed, and on the choice of adequate regimens or the number of cycles/duration if adjuvant treatment is given. In the recent Checkmate 274 trial, adjuvant nivolumab improved disease-free survival especially in the subgroup of patients treated with neoadjuvant chemotherapy and patients with pN+ disease, and the OS data of the study is eagerly awaited [14]. Although the subgroup of patients with ypN+ disease was not specifically evaluated in the trial, the role of adjuvant immunotherapy in these patients warrants further investigation.
The importance of PLND in bladder cancer is well-described. Multiple retrospective studies reported the association between the number of removed lymph nodes and favorable survival outcomes previously [3,15]. In our study, ≥ 20 lymph nodes removed were associated with greater RFS. These findings might be translated into the prognostic importance of extensive tumor removal. However, the exact extent of lymph node dissection and its prognostic implication have yet to be determined [9,16]. A previous phase 3 trial failed to show improved survival outcomes with extended lymphadenectomy; however, patients who experienced neoadjuvant chemotherapy were not included in the study [17]. The result of the ongoing Southwest Oncology Group (SWOG) 1011 trial which includes neoadjuvant chemotherapy-treated patients might assist in answering this important clinical question [18].
Data on the role of adjuvant radiotherapy in patients with pN+ disease after neoadjuvant chemotherapy is scarce. Considering that the most common relapse pattern was distant metastasis in our study and that previous studies expectedly failed to show improvements in the distant metastasis-free survival with radiotherapy [19,20], additional radiotherapy might not provide clinical benefit in these patients.
In this study, preoperative imaging yielded a low sensitivity (63.5%) for the identification of pathological lymph node positivity. In addition, only 25% of patients had concordant clinical and pathological lymph node stage. These findings are in line with previous studies that reported unsatisfactory predictive value of clinical staging with accuracy for lymph node metastases of 54%–83% [21–24]. Recent studies showed an improved diagnostic yield of imaging with alternative methods such as diffusion-weighted magnetic resonance imaging [25,26]. Further studies might help to identify these patients with poor prognoses preoperatively.
Although it is beyond the scope of our study, given the poor RFS after surgery and the limited response to chemotherapy, the risks, and benefits of surgical treatment should be carefully weighed in these patients. However, since the possibility of achieving long-term survival cannot be completely excluded even in patients with M1a disease [27], careful treatment decisions should be made. Response to neoadjuvant chemotherapy might help in appropriate patient selection [28–30].
This study is limited by its single-centred, retrospective nature. The effect from unmeasured confounding factors such as performance status could affect survival outcomes. In addition, owing to the limited number of patients who received and completed adjuvant chemotherapy, the effectiveness of adjuvant chemotherapy might be attenuated. However, these results are likely to reflect the real-world outcomes and feasibility of adjuvant chemotherapy, including the high recurrence rates and poor tolerability during treatment. In addition, although no patients were considered positive for M1a lymph node metastases at the time of surgery, independent radiologist review identified those with distant lymph node metastases, ascribing to the low accuracy of clinical staging. This could attribute to the poor outcome and lack of observed benefit of adjuvant chemotherapy in our study. However, subgroup analysis excluding those with ypM1a disease consistently showed no survival benefit with the addition of the adjuvant chemotherapy. Despite these limitations, our study is one of the first which were dedicated to patients with ypN+ disease. Along with the long-term follow-up duration, detailed analysis on the effectiveness and tolerability of adjuvant chemotherapy, and utility of clinical staging, the results of our study would provide useful information for the management of these patients where no standardized treatment approach has been made due to the lack of supporting evidence.
In conclusion, patients with ypN+ disease after neoadjuvant chemotherapy followed by surgery showed high recurrence rates with limited survival outcomes. Little benefit was observed with the addition of adjuvant chemotherapy.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board (IRB No. 2021-0128) of the Asan Medical Center and conducted in accordance with the Declaration of Helsinki. The IRB granted an informed-consent waiver for this retrospective study. Author Contributions Conceived and designed the analysis: Yoon S, Lee JL. Collected the data: Jeong H, Park KJ, Lee Y, Kim HD, Kim JH, Yoon S, Hong B, Lee JL. Contributed data or analysis tools: Jeong H, Park KJ, Yoon S, Hong B, Lee JL. Performed the analysis: Jeong H. Wrote the paper: Jeong H, Park KJ, Lee Y, Kim HD, Kim JH, Yoon S, Hong B, Lee JL. Fig. 1Kaplan-Meier plot: (A) recurrence-free survival and overall survival in the entire study population, (B) recurrence-free survival by adjuvant chemotherapy, (C) overall survival by adjuvant chemotherapy. Numbers shown in parentheses indicate a 95% confidence interval. 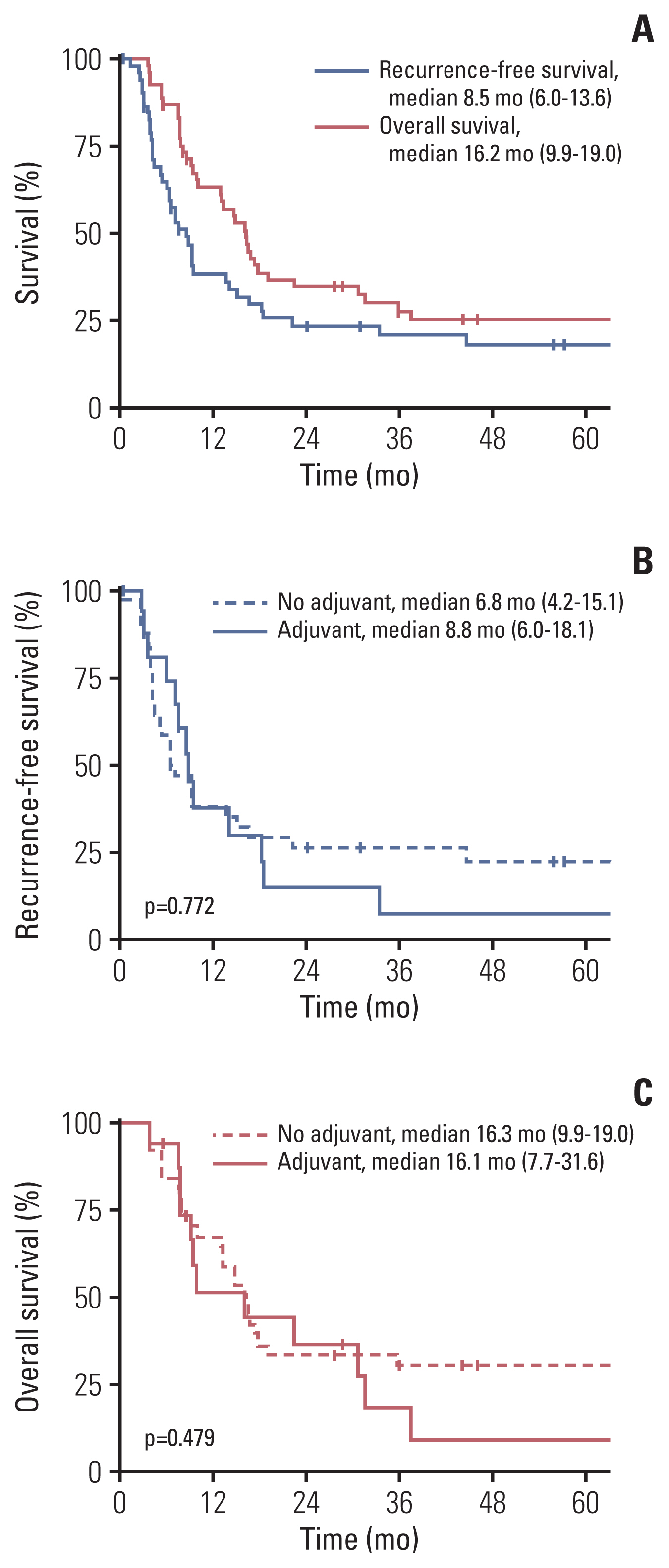
Table 1Baseline characteristics Table 2Summary of adjuvant chemotherapy References1. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogel-zang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66.
2. Sui W, Lim EA, Joel Decastro G, McKiernan JM, Anderson CB. Use of adjuvant chemotherapy in patients with advanced bladder cancer after neoadjuvant chemotherapy. Bladder Cancer. 2017;3:181–9.
3. Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22:2781–9.
4. Cha EK, Sfakianos JP, Sukhu R, Yee AM, Sjoberg DD, Bochner BH. Poor prognosis of bladder cancer patients with occult lymph node metastases treated with neoadjuvant chemotherapy. BJU Int. 2018;122:627–32.
5. Galsky MD, Stensland KD, Moshier E, Sfakianos JP, McBride RB, Tsao CK, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34:825–32.
6. Seisen T, Jamzadeh A, Leow JJ, Roupret M, Cole AP, Lipsitz SR, et al. Adjuvant chemotherapy vs observation for patients with adverse pathologic features at radical cystectomy previously treated with neoadjuvant chemotherapy. JAMA Oncol. 2018;4:225–9.
7. Zargar-Shoshtari K, Kongnyuy M, Sharma P, Fishman MN, Gilbert SM, Poch MA, et al. Clinical role of additional adjuvant chemotherapy in patients with locally advanced urothelial carcinoma following neoadjuvant chemotherapy and cystectomy. World J Urol. 2016;34:1567–73.
8. Martinez Chanza N, Werner L, Plimack E, Yu EY, Alva AS, Crabb SJ, et al. Incidence, patterns, and outcomes with adjuvant chemotherapy for residual disease after neoadjuvant chemotherapy in muscle-invasive urinary tract cancers. Eur Urol Oncol. 2020;3:671–9.
9. Hwang EC, Sathianathen NJ, Imamura M, Kuntz GM, Risk MC, Dahm P. Extended versus standard lymph node dissection for urothelial carcinoma of the bladder in patients undergoing radical cystectomy. Cochrane Database Syst Rev. 2019;5:CD013336.
10. McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology. 2010;254:31–46.
11. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
12. Kassouf W, Agarwal PK, Grossman HB, Leibovici D, Munsell MF, Siefker-Radtke A, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urology. 2009;73:147–52.
13. Donat SM, Shabsigh A, Savage C, Cronin AM, Bochner BH, Dalbagni G, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009;55:177–85.
14. Bajorin DF, Witjes JA, Gschwend J, Schenker M, Valderrama BP, Tomita Y, et al. First results from the phase 3 CheckMate 274 trial of adjuvant nivolumab vs placebo in patients who underwent radical surgery for high-risk muscle-invasive urothelial carcinoma (MIUC). J Clin Oncol. 2021;39(6_Suppl):391.
15. Wright JL, Lin DW, Porter MP. The association between extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Cancer. 2008;112:2401–8.
16. Jensen JB, Ulhoi BP, Jensen KM. Extended versus limited lymph node dissection in radical cystectomy: impact on recurrence pattern and survival. Int J Urol. 2012;19:39–47.
17. Gschwend JE, Heck MM, Lehmann J, Rubben H, Albers P, Wolff JM, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. 2019;75:604–11.
18. S1011 standard or extended pelvic lymphadenectomy in treating patients undergoing surgery for invasive bladder cancer [Internet]. Bethesda, MD: US National Library of Medicine; 2010. [cited 2021 Mar 2]. Available from: https://ClinicalTrials.gov/show/NCT01224665
19. Zaghloul MS, Christodouleas JP, Smith A, Abdallah A, William H, Khaled HM, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg. 2018;153:e174591.
20. Iwata T, Kimura S, Abufaraj M, Janisch F, Karakiewicz PI, Seebacher V, et al. The role of adjuvant radiotherapy after surgery for upper and lower urinary tract urothelial carcinoma: A systematic review. Urol Oncol. 2019;37:659–71.
21. Mehrsai A, Mansoori D, Taheri Mahmoudi M, Sina A, Seraji A, Pourmand GH. A comparison between clinical and pathologic staging in patients with bladder cancer. Urol J. 2004;1:85–9.
22. Husband JE. Computer tomography and magnetic resonance imaging in the evaluation of bladder cancer. J Belge Radiol. 1995;78:350–5.
23. Horn T, Zahel T, Adt N, Schmid SC, Heck MM, Thalgott MK, et al. Evaluation of computed tomography for lymph node staging in bladder cancer prior to radical cystectomy. Urol Int. 2016;96:51–6.
24. Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163:1693–6.
25. Thoeny HC, Froehlich JM, Triantafyllou M, Huesler J, Bains LJ, Vermathen P, et al. Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology. 2014;273:125–35.
26. Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol. 2010;54:358–64.
27. Sweeney P, Millikan R, Donat M, Wood CG, Radtke AS, Pettaway CA, et al. Is there a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J Urol. 2003;169:2113–7.
28. Otto T, Krege S, Suhr J, Rubben H. Impact of surgical resection of bladder cancer metastases refractory to systemic therapy on performance score: a phase II trial. Urology. 2001;57:55–9.
|
|
||||||||||||||||||||||||||||||||||||||||||||||