AbstractPurposeThere are unmet needs associated with the current treatment strategies for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) due to the poor treatment outcomes of these strategies. Roflumilast, a selective phosphodiesterase-4 inhibitor used for treating chronic obstructive pulmonary disease, is effective against B-cell malignancy via phosphoinositide 3-kinase (PI3K)–activity suppression. We analyzed the effects of roflumilast combined with ESHAP (etoposide, cisplatin, methylprednisolone, and cytarabine) chemotherapy in experimental and clinical settings.
Materials and MethodsAn in vitro study using lymphoma cell lines and a pilot study on relapsed/refractory DLBCL patients were conducted to investigate the effects and mechanism of the combination of roflumilast and chemotherapy. The complete response (CR), overall response rate (ORR), and 1-year progression-free survival (PFS) were analyzed.
ResultsWe found that roflumilast is efficient when combined with other chemotherapy drugs, especially cytarabine. Synergistic effects between these two drugs influence the translation of mammalian target of rapamycin and myeloid cell leukemia 1, resulting in apoptosis and inhibition of B-cell lymphoma proliferation. In clinical setting, the roflumilast group showed better rates of CR (46.2% vs. 34.6%), ORR (76.9% vs. 53.8%), and 1-year PFS (50.0% vs. 25.9%) compared with the control group, though not statistically significant. The roflumilast group showed a higher incidence of asthenia and gastrointestinal adverse events. However, grade 3 or 4 adverse events were similar in both groups.
IntroductionDiffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL) among adults. It accounts for about 31% of all NHL cases in Western countries and South Korea [1,2]. Since the introduction of R-CHOP chemotherapy, which involves the use of the monoclonal antibody rituximab in addition to the previous CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy, treatment outcomes have dramatically improved [3]. However, 35%–40% patients are refractory or relapsed after R-CHOP chemotherapy, and in such cases the treatment outcome is extremely poor [4]. Salvage chemotherapy followed by autologous hematopoietic stem cell transplantation (ASCT) has been widely used for relapsed/refractory disease, but there is no established consensus regarding the effective regimen yet [5–7].
Roflumilast is an anti-inflammatory drug that selectively inhibits phosphodiesterase 4 (PDE4) enzymes. It is used to reduce the number of episodes and aggravation of symptoms in people with chronic obstructive pulmonary disease (COPD) by controlling inflammatory conditions [8]. Previous studies have shown that this PDE4 inhibitor is also effective against B-cell malignancy through the phosphoinositide 3-kinase (PI3K)/AKT/mTOR signaling pathway and vascular endothelial growth factor (VEGF)–related angiogenesis [9,10]. Based on these results, several clinical trials are in progress to improve the results of DLBCL treatment by adding roflumilast to current chemotherapy regimens. However, these trials are in the early stage and the rationale for these trials is still limited. Moreover, when roflumilast is combined with conventional chemotherapy, which drug induces the greatest synergistic effect in combination with roflumilast remains unclear.
We aimed to determine the effects of combining roflumilast with several chemotherapy agents. Based on our in vitro findings, we conducted a pilot study to confirm the efficacy and safety of roflumilast combined with salvage chemotherapy in patients with relapsed/refractory DLBCL.
Materials and Methods1. Cell culture, antibodies, and reagentsThree PDE4B-high cell lines–namely OCI-Ly1 (RRID: CVCL_1879), OCI-Ly10 (RRID: CVCL_8795), and Ramos (RRID: CVCL_0597)–were kindly provided by Dr. Ricardo Aguiar, University of Texas, Health Science Center at San Antonio (UTHSCSA). All cell lines were authenticated using short tandem repeat profiling (Cosmogen Tech, Seoul, Korea) within the last 3 years and all experiments were performed using mycoplasma-free cells. Cells were cultured in RPMI medium (GIBCO) supplemented with 10% fetal bovine serum (Hyclone, Radnor, PA), 1% N-2-hydroxyethylpiperanzine-N’-2-ethanesulfonic acid (HEPES), 1% L-glutamine, and 1% penicillin/streptomycin (PENSTREP) at 37°C in a 5.0% CO2 incubator. The primary and secondary antibodies used for Western blot were as follows: anti-phospho-4EBP1 (1:1,000, Cat#9459, Cell Signaling Technology, Danvers, MA), myeloid cell leukemia 1 (MCL1; 1:1,000, sc-819, Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (1:5,000, sc-47778, Santa Cruz Biotechnology), HRP-conjugated anti-rabbit (1:10,000, A120-101p, Bethyl, Montgomery, AL), and anti-mouse secondary antibodies (1:10,000, A90-116p-33, Bethyl). The reagents used in this study were cytarabine (JW-Life Science, Dangjin, Korea), forskolin (cat# BML-CN100-0010, Enzo Life Science, Farmingdale, NY), roflumilast (cat# 2675, BioVision, Milpitas, CA), and JC-1 dyes (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, KA1-324, Abnova, Walnut, CA).
2. Cell viability assayThe three PDE4B-high cell lines (Ly1, Ly10, and Ramos) were plated in 96-well micro plates at a density of 3×105 cells/well each and treated with cytarabine (0, 0.5, 1, and 2 μM), forskolin/roflumilast (0, 10, 20, and 40 μM/40 μM), or their combination as indicated for 48 hours. Cell viability was estimated using the Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS; Promega, Madison, WI). The MTS solution was added to the cells at 30 μL per well and the plate was incubated for 2 hours. The absorbance was recorded at 450 nm with a GloMax Microplate multi-reader (Promega).
3. ImmunoblottingProtein levels were analyzed using western blot. Briefly, cells were harvested and washed with 1× phosphate buffered saline and lysed in a lysis buffer (RIPA buffer), Na-vanadate (1 mM), β-glycerol phosphate (50 mM), protease inhibitor (cat#786-108, G-biosciences, St. Louis, MO), EDTA (5 mM), β-mercaptoethanol (cat#41300000-1, 142 mM, BioWORLD, Dublin, OH). The cell lysate was mixed with 5× sample buffer and the mixture was boiled at 100°C for 10 min. Subsequently, the samples were loaded on 12% and 15% polyacrylamide gels. Proteins were transferred to membranes using Mini Trans-Blot Cell and Criterion Blotter (Bio-Rad, Hercules, CA) and the membranes were blocked in 1% BSA (bovine serum albumin, cat#160069, MP Biomedicals, Santa Ana, CA) dissolved in Tris-buffered saline containing 0.1% Tween 20 (TBST). The membranes were probed by indicated primary antibodies and incubated on a rotator at 4°C overnight. Then, the membranes were washed for 5 minutes in TBST and treated with anti-mouse or -rabbit secondary antibodies for 1 hour at room temperature. After washing three times for 10 minutes each with TBST, proteins were detected using a chemiluminescent substrate (EZ west Lumi Plus, ATTO, Tokyo, Japan) and visualized on a chemiluminescence Imaging system (Luminograph II, ATTO).
4. Analysis of mitochondrial membrane potentialThe cell lines (Ly1, Ly10, and Ramos) at 1.0×106 cells/well were treated with forskolin/roflumilast (20 μM/20 μM), cytarabine (0.5 μM), or their combination and incubated for 48 hours. Before staining, JC-1 dye was diluted 1:10 by RPMI medium. Then, 20 μL of JC-1 was added to each well and incubated for 20 minutes at 37°C. Using a fluorescence microscope (Olympus, Tokyo, Japan), JC-1-stained cells were detected under the 520 nm optical filter. All stained cells and red-stained cells were counted and the ratio of red-stained cells to all stained cells was calculated using Microsoft Excel.
5. Clinical study designFor the clinical evaluation of our regimen, a pilot study was conducted. According to our center’s policy, relapsed/refractory NHL patients were treated with ESHAP (etoposide, cisplatin, methylprednisolone, and cytarabine), DHAP (dexamethasone, cytarabine, and cisplatin), or ICE (ifosfamide, carboplatin, and etoposide) chemotherapy regimens. Based on the findings our in vitro experiments, we selected ESHAP regimen in combination with roflumilast for clinical evaluation.
From September 2017 to May 2018, patients who received ESHAP chemotherapy combined with roflumilast were enrolled. Patients received roflumilast 500 mg once daily from the beginning of the first cycle of ESHAP chemotherapy to the end of the treatment. Dose modification including roflumilast and supportive care for toxicity were performed at the discretion of the investigator. For comparative analysis, medical records of patients who received original ESHAP chemotherapy from 2013 to 2018 were retrospectively reviewed. Criteria for eligibility included histologically confirmed DLBCL, which was relapsed/refractory after R-CHOP chemotherapy; age ≥ 18 years; and Eastern Cooperative Oncology Group (ECOG) performance score of 0–2. Patients with primary DLBCL of the central nervous system, human immunodeficiency virus infection, or post-transplant lymphoproliferative disorder were excluded.
Patient records of diagnosis, treatment outcomes, and safety were collected and analyzed. Treatment response was assessed using the National Cancer Institute (NCI)–sponsored Working Group guidelines. Safety was evaluated by reviewing the incidence, severity, relationship, and type of adverse events that occurred during the treatment and follow-up periods using the revised NCI Common Terminology Criteria for Adverse Events, ver. 4.03.
6. Statistical analysisCombination index (CI) values were estimated using the CompuSyn software (ver. 1.0, ComboSyn, Inc., Paramus, NJ). We used the percent inhibition of cell viability resulting from drug combinations (fraction affected, Fa) versus individual agents. To identify statistically significant differences (p < 0.05), one-way ANOVA was performed using Excel 2007 (Microsoft) and Prism (GraphPad, San Diego, CA).
Chi-square test or Fisher exact test were used to compare categorical variables. Continuous variables were examined using t test or u test depending on data distribution. Progression-free survival (PFS) was calculated from the date of treatment start to the date of documented disease progression, the last follow-up visit, or death if the disease had not progressed until the time of investigation. Overall survival (OS) was calculated from the date of salvage chemotherapy start until either death or the date last known to be alive. PFS and OS curves were constructed using the Kaplan-Meier method. For all analyses, p < 0.05 was considered statistically significant. SPSS ver. 22.0 (IBM Corp., Armonk, NY) was used for all clinical data analyses.
Results1. In vitro synergistic effect of roflumilast and commonly used chemotherapy agents on cell viabilityTo identify which of the drugs used for salvage chemotherapy of DLBCL had synergistic effect with roflumilast, the Ly1 cell line was treated with roflumilast (0 or 40 μM) in combination with forskolin (0, 10, 20, or 40 μM) and one of the six chemotherapy agents (cytarabine, cisplatin, mitoxantrone, etoposide, carboplatin, ifosfamide) commonly used for salvage chemotherapy of DLBCL. The treatment was conducted for 48 hours and the MTS assay was performed to estimate cell viability. The data were normalized to the respective values in vehicle-treated cells (no forskolin, roflumilast, or chemotherapy agents). Among these six drugs, cytarabine showed statistically significant synergistic effect when combined with roflumilast. In the case of ifosfamide and carboplatin, cell viability tended to increase when used alone, but it tended to decrease when used with roflumilast (Fig. 1).
2. Synergistic effect of roflumilast and cytarabine on inhibition of B-cell lymphoma viabilityTo confirm the efficacy of the combinatorial effects of cytarabine and roflumilast on cell viability, three cell lines (Ly1, Ly10, and Ramos) were treated with forskolin/roflumilast, cytarabine, or their combination for 48 hours, and the MTS assay was performed. Treatment with forskolin/roflumilast or cytarabine alone suppressed cell viability, but their combinatorial treatment inhibited cell viability to a much greater extent when compared with each single treatment (Fig. 2A). To quantitatively determine the interactions between forskolin/roflumilast and cytarabine or the drug-induced cytotoxic synergy, CI values were calculated. These values indicated that combinatorial treatment with forskolin/roflumilast and cytarabine had strong and very strong synergy in Ly1 and Ly10, respectively, but had moderate synergy in Ramos (Fig. 2B). In conclusion, co-administration of cytarabine and roflumilast has synergistic effects on the inhibition of B-cell lymphoma proliferation than single administration of either drug.
3. Synergistic effects between roflumilast and cytarabine influence mammalian target of rapamycin signaling and MCL1 levelsPrevious studies have shown that PDE4 inhibitors and cytarabine inhibit mammalian target of rapamycin (mTOR) signaling [11,12]. To investigate the combinatorial effects of forskolin/roflumilast and cytarabine on the mTOR signaling pathway and its downstream target MCL1, three B-cell lymphoma cell lines—Ly1, Ly10, and Ramos—were treated with forskolin/roflumilast, cytarabine, or a combination of the two. Western blot analysis of the downstream substrates of the mTOR signaling pathway was then performed. Combinatorial treatment using forskolin/roflumilast and cytarabine markedly downregulated phospho-4EBP1 and MCL1 compared to the individual treatments (Fig. 3). These results suggest that co-administration of forskolin/roflumilast and cytarabine is more effective for targeting the mTOR/MCL1 pathway in B-cell lymphoma.
4. The combinatorial effects of roflumilast and cytarabine on mitochondrial membrane potential in B-cell lymphomaGiven that pro-survival MCL1 is synergistically repressed by forskolin/roflumilast and cytarabine, we assessed if this combination influences mitochondrial membrane potential (MMP). To measure MMP, the three cell lines—Ly1, Ly10, and Ramos—were treated with forskolin/roflumilast, cytarabine, or their combination for 48 hours. MMP was then assessed using JC-1 fluorescence staining. At high MMP, JC-1 forms an aggregation and emits red fluorescence, whereas at low MMP, it exists as a monomer and emits green fluorescence. As shown in Fig. 4A, forskolin/roflumilast or cytarabine alone had little impact on green fluorescence intensity compared with the control group in all three cell lines. However, combinatorial treatment decreased red fluorescence intensity and increased green fluorescence intensity in comparison with the individual treatments. To quantify the extent of apoptosis, all stained cells were counted and the ratio of the number of red-stained (live) cells to the number of all stained cells was determined. Quantification analysis confirmed that combinatorial treatment significantly reduced red-stained cells than individual treatments (Fig. 4B). These results indicate that co-administration of forskolin/roflumilast and cytarabine induces more intensive apoptosis in B-cell lymphoma cells than their single administration by disrupting MMP.
5. Characteristics of patients in the pilot studyDuring the study period, 13 patients were enrolled in the roflumilast group and received ESHAP chemotherapy in combination with roflumilast as a salvage treatment. As a control group, medical records of 26 patients with relapsed/refractory DLBCL treated with ESHAP regimen were collected. Patient characteristics of the two groups at diagnosis and occurrence of relapsed/refractory disease are presented in Table 1. There were minor differences regarding patient characteristics between the two groups; however, these differences were not significant. The median age of the roflumilast group was 10 years greater than that of the control group (69 years vs. 59 years, p=0.093). In addition, the proportion of patients older than 65 years was higher in the roflumilast group (69.2% vs. 42.3%); however, this was not statistically significant. About 70% of the patients showed ECOG performance score of 0 or 1 before salvage chemotherapy. There was no significant difference in International Prognostic Index risk score and overall response rate (ORR) for R-CHOP chemotherapy between the two groups. Two of the 13 patients (15.4%) in the roflumilast group and eight of the 26 patients (30.8%) in the control group had refractory disease.
6. Survival and response to ESHAP chemotherapy combined with roflumilastORR of the roflumilast group (76.9%) was higher than that of the control group (53.8%). Six patients (46.2%) of the roflumilast group and nine patients (34.6%) of the control group achieved complete remission. More patients in the control group did not respond to ESHAP chemotherapy (23.1% vs. 42.3%). For median follow-up duration of 12.9 months, 46.2% of the patients from the roflumilast group and 76.9% of the control group experienced relapse or refractoriness to ESHAP chemotherapy (p=0.055). In the analysis of disease progression, median PFS showed no significant difference in the two groups (6.9 months vs. 5.7 months). For 1-year PFS rate, the roflumilast group showed better, though not statistically significant, results (50.0% vs. 25.9%) compared with the control group. In the survival analysis, there was no significant difference between the two groups regarding median OS (13.4 months vs. 12.9 months) and 1-year OS rate (53.8% vs. 53.8%). Further results of survival are shown in Table 2 and Figs. 5, 6.
7. Toxicity of ESHAP chemotherapy combined with roflumilastWe collected data on the adverse events associated with 50 cycles of chemotherapy for the roflumilast group and 54 cycles for the control group. We could collect adverse event records from 54 of 90 total cycles in the control group. Hematologic adverse events were not significantly different between the two groups. Hepatopathy (18.0% vs. 5.6%) and renal failure (16.0% vs. 1.9%) were more common in the roflumilast group, but there was no grade 3 or 4 event. The most common non-hematologic adverse events observed among the patients in the roflumilast group were asthenia, anorexia, nausea, and dizziness; most events were of grade 1 or 2. The most common non-hematologic adverse events of grade 3 or higher observed among the patients in the roflumilast group were anorexia and diarrhea. Adverse events leading to treatment-related mortality were observed in one case (7.7%) in the roflumilast group and in three cases (11.5%) in the control group. The occurrence of adverse events according to treatment group is summarized in Table 3.
DiscussionPatients with relapsed/refractory DLBCL have dismal prognosis with limited therapeutic options. Treatment options in this setting include gemcitabine and/or platinum-based therapy as well as bendamustine and rituximab for transplant ineligible patients. However, there is no standard salvage chemotherapy regimen, which is globally accepted [5–7]. Recently, novel drugs with mechanisms of action that are different from those of classic drugs were introduced for relapsed/refractory DLBCL. Polatuzumab vedotin, a CD 79b-targeted antibody-drug conjugate, combined with bendamustine and rituximab, was approved by the US Food and Drug Administration (FDA), and bispecific T-cell engagers (BiTEs), such as blinatumomab, showed promising results in phase II studies; further studies are ongoing to identify the optimal role of these drugs in relapsed/refractory DLBCL treatment. CD19-directed chimeric antigen receptor (CAR) T-cell therapy also showed promising results; however, the clinical application of these novel drugs is limited because of the high costs associated with their use and the need for specialized centers for their administration [13–15]. Therefore, combinations of existing drugs may serve as good alternatives to currently available therapeutic strategies and can lead to better patient outcomes.
Roflumilast is a drug approved by the US FDA for COPD treatment [8]. It mainly works by inhibiting PDE4, which regulates the synthesis and degradation of cyclic adenosine monophosphate (cyclic-AMP) [16,17]. PDE4 is also known to be involved in B-cell lymphoma pathogenesis via two mechanisms: the PI3K/AKT pathway of B-cell receptor (BCR) signals and angiogenesis-related microenvironment. In the former case, subsequent studies have shown that cyclic-AMP downmodulates the PI3K/AKT pathway of BCR signals to promote cell death [9]. In the latter case, in B-cell lymphoma microenvironments with high PDE4B levels, cyclic-AMP is hydrolyzed to AMP, resulting in angiogenesis enhanced by higher AKT-induced VEGF expression and excessive secretion in the tumor milieu [10]. Shipp et al. [18] first suggested the association between PDE4B and B-cell lymphoma by conducting a genome study in which they showed that high PDE4B expression distinguishes curable DLBCL from fatal DLBCL. The association between high PDE4B expression and poor DLBCL outcome was subsequently confirmed in larger independent series [9,19,20].
In this study, we combined roflumilast with drugs commonly used in salvage chemotherapy of relapsed/refractory DLBCL to confirm if this combination is effective in treating DLBCL. Among the various combination tests, we confirmed that co-administration of cytarabine and roflumilast has synergistic effect on inhibiting the growth of B-cell lymphoma and induces more intensive apoptosis than single administration. Cytarabine is a pyrimidine nucleoside analog that incorporates into human DNA and kills cells by disturbing DNA and RNA synthesis at the S phase of the cell cycle. In hematologic malignancies, cytarabine also reduces AKT phosphorylation and consequently suppresses mTOR, which has an anti-cancer effect by inhibiting the PI3K/AKT/mTOR pathway [11,21,22]. In our in vitro research, roflumilast was found to be the most effective when combined with cytarabine among several drugs. It is thought that when roflumilast and cytarabine are combined, dual inhibition occurs for the same PI3K/AKT/mTOR pathway, resulting in a synergistic effect.
In the major salvage regimens of DLBCL, cytarabine is included in the ESHAP and DHAP regimens. Of these, ESHAP is mainly used as the first salvage regimen in our center. In this study, the roflumilast group showed a better result regarding ORR than the control group, but it was not statistically significant because of small patient number. This result may have been affected by higher proportion of patients in the control group who had R-CHOP refractory disease. Nevertheless, it is a meaningful result considering that the median age of patients in the roflumilast group was 10 years higher than that of patients in the historical control group. Although direct comparisons are not possible, the ORR of the roflumilast plus ESHAP regimen is higher than that observed in a previous ESHAP trial. Velasquez et al. [23] reported an ORR of 53.1% in relapsed/refractory DLBCL patients treated with ESHAP. Ezzat et al. [24] and Choi et al. [25] reported an ORR of about 70% for ESHAP treatment in relapsed/refractory NHL; however, because these two studies included indolent lymphomas, if the disease is limited to DLBCL, the response rates are expected to decrease. The GEL/TAMO group combined rituximab with ESHAP and reported an ORR of 67%. This result is also inferior to that of our study [23–26].
The difference in the median PFS and OS was not clear, but the survival graph showed an increasing gap between the two groups after 8 months in the PFS curve and after 17 months in the OS curve. The two groups showed a difference in 1-year PFS (50% vs. 25.9%); thus, it seems that roflumilast combination affects the resistance mechanism and the effect of long-term follow-up can be expected. One patient in the roflumilast group received ASCT after salvage chemotherapy and has been in remission status for 27 months until now.
Overall adverse events occurred more in the roflumilast group; however, adverse events of grade 3 or higher or treatment-related mortality were similar or more in the control group. Although the complete adverse events data of the control group was not obtained because of the retrospective nature of data collection, it appeared that more hepatopathy, renal failure, anorexia, and gastrointestinal symptoms occurred in the roflumilast group, which requires more careful management compared to conventional treatments.
Several past studies have shown the antitumor activity of roflumilast. They reported positive results for B-cell malignancy and solid cancer such as lung cancer and ovarian cancer [27,28]. However, most were in vitro studies and studies in clinical settings were rare. Recently, Kelly et al. [29]reported a phase Ib clinical trial with advanced B-cell malignancies. They described favorable responses with a combination of roflumilast and prednisolone and successful clinical repurposing of roflumilast in B-cell malignancies. Three DLBCL patients were included in this study with one partial response. Although the number of patients involved is very small, it is meaningful as the first study using roflumilast for hematologic malignancy [29,30]. Based on these results, a clinical trial that combines roflumilast and R-CHOP chemotherapy in patients with newly diagnosed high-risk DLBCL is ongoing (NCT03458546).
Our study had several limitations. First, owing to the small number of patients and single-center setting, the results carried insufficient statistical power. Second, in clinical settings, most chemotherapies are performed in a multi-drug combination regimen, whereas our in vitro study was conducted with combinations involving a single drug. We speculate that real world data differs from experimental results in this context.
In conclusion, our study experimentally proved that roflumilast, a PDE4 inhibitor that has recently gained increasing interest in the field of DLBCL treatment, is efficient when combined with other chemotherapy drugs, especially cytarabine. A pilot study conducted on the basis of the findings of in vitro studies suggested that the combination of roflumilast and a chemotherapeutic regimen including cytarabine may be clinically effective. We believe that these results will contribute to the improvement of DLBCL treatment outcome. Further, we look forward to the results of ongoing front-line combination and the beginning of further studies in the future.
NotesEthical Statement This study was conducted according to the Declaration of Helsinki, it was approved by the Institutional Review Board of Pusan National University Hospital (2006-020-092), and informed consent was obtained from all individual participants included in the study. Fig. 1Synergistic effect of roflumilast with chemotherapy drugs. A PDE4B-high cell line, OCI-Ly1, was treated with was treated with roflumilast (0 or 40 μM) in combination with forskolin (0, 10, 20, or 40 μM) and one of the six chemotherapy agents (cytarabine, cisplatin, mitoxantrone, etoposide, carboplatin, ifosfamide) that are commonly used for salvage chemotherapy for diffuse large B-cell lymphoma. The treatment was conducted for 48 hours, as indicated and the MTS assay were performed to measure cell viability. The results are presented as mean±standard deviation and are representative of three independent experiments (*p < 0.05, One-way ANOVA). Frs, forskolin; Rf, roflumilast. 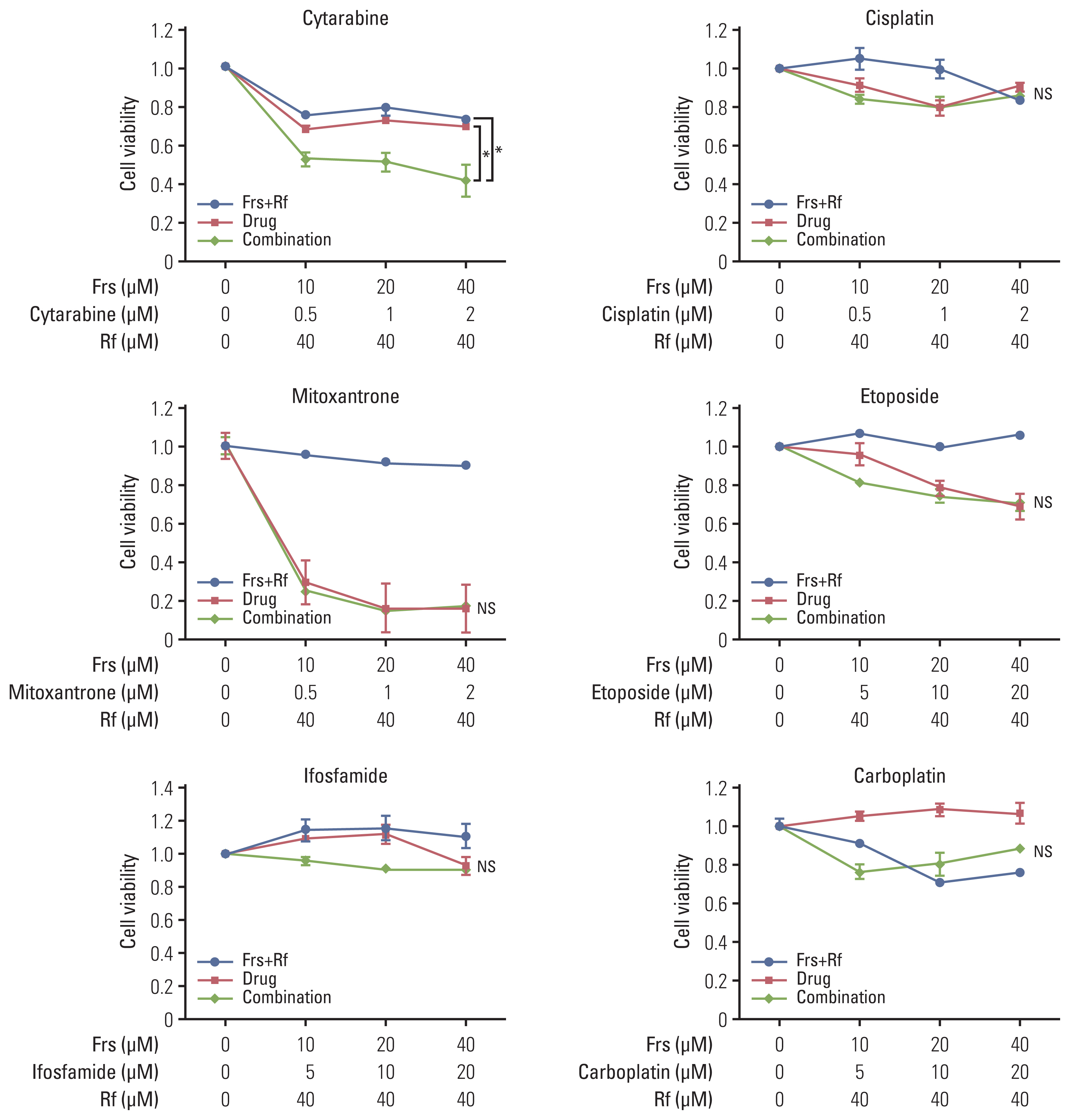
Fig. 2Combination of roflumilast and cytarabine have synergistic effects on cell death. (A) Three PDE4B-high cell lines (Ly10, Ly1, and Ramos) were treated with forskolin (0, 10, 20, or 40 μM), cytarabine (0, 0.5, 1, or 2 μM) and roflumilast (0 or 40 μM) for 48 hours, as indicated and the MTS assay were performed to measure cell viability. The results are presented as mean±standard deviation and are representative of three independent experiments (*p < 0.05, One-way ANOVA). (B) To evaluate the levels of drug synergy, combination index (CI) values were estimated using the CompuSyn software. The percent inhibition (fraction affected, Fa) from drug combinations was compared with that from individual drugs. Synergy levels are as follows: < 0.1 very strong synergy; 0.1–0.3, strong synergy; 0.3–0.7, synergy; 0.7–0.85, moderate synergy; 0.85–0.90, slight synergy; 0.90–1.10, nearly additive; 1.10–1.20, slight antagonism; 1.20–1.45, moderate antagonism; and 1.45–3.30, antagonism. Cyt, cytarabine; Frs, forskolin; Rf, roflumilas. 
Fig. 3Roflumilast and cytarabine regulate the translation of mammalian target of rapamycin and myeloid cell leukemia 1 (MCL1). p4EBP1 and MCL1 protein levels were analyzed by western blotting. β-Actin was used as an internal control. Cyt, cytarabine; Frs, forskolin; Rf, roflumilast. 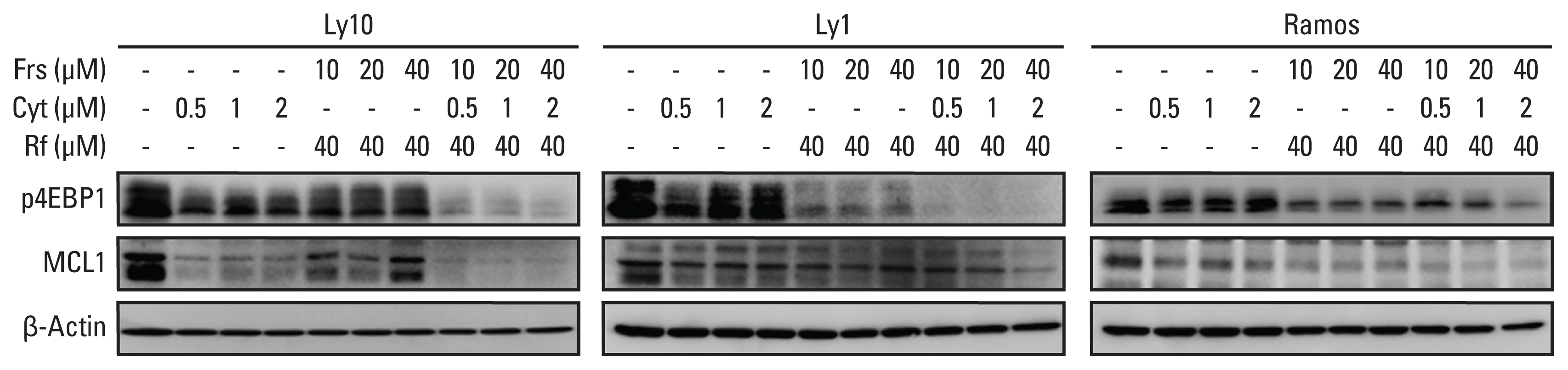
Fig. 4Synergistic effect of roflumilast and cytarabine on mitochondrial membrane potential. (A) To identify the potential role of disruption of the mitochondrial membrane potential (MMP) in apoptosis, an MMP assay was performed. First, all three cell lines (Ly1, Ly10, and Ramos) were treated with forskolin/roflumilast (40 μM), cytarabine (0.5 μM), or their combination for 48 hours and stained with the membrane-permeant dye JC-1. Subsequently, mitochondrial states were observed using fluorescence microscopy. Red-stained cells indicate live cells and green-stained cells indicate dead cells. (B) To quantify the number of cells with MMP disruption, all stained cells were counted and the ratio of red-stained cells to all stained cells was determined. The ratio was normalized to the control (DMSO) group. The results are presented as mean±standard deviation and are representative of three independent experiments (*p < 0.05, One-way ANOVA). Frs, forskolin; Rf, roflumilast. 
Fig. 5Kaplan-Meier curves showing the progression-free survival (PFS) (A) and overall (OS) (B) of the patients. 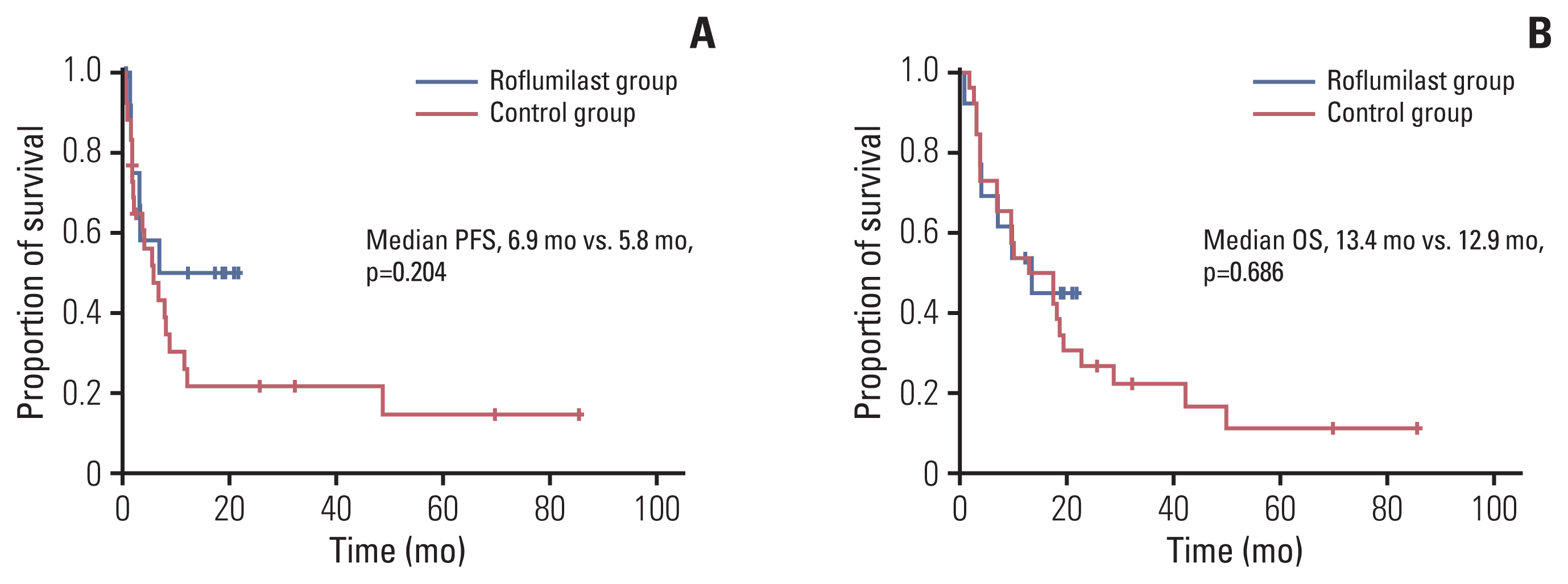
Fig. 6Swimmer plot of 13 patients who received ESHAP (etoposide, cisplatin, methylprednisolone, and cytarabine) chemotherapy combined with roflumilast. 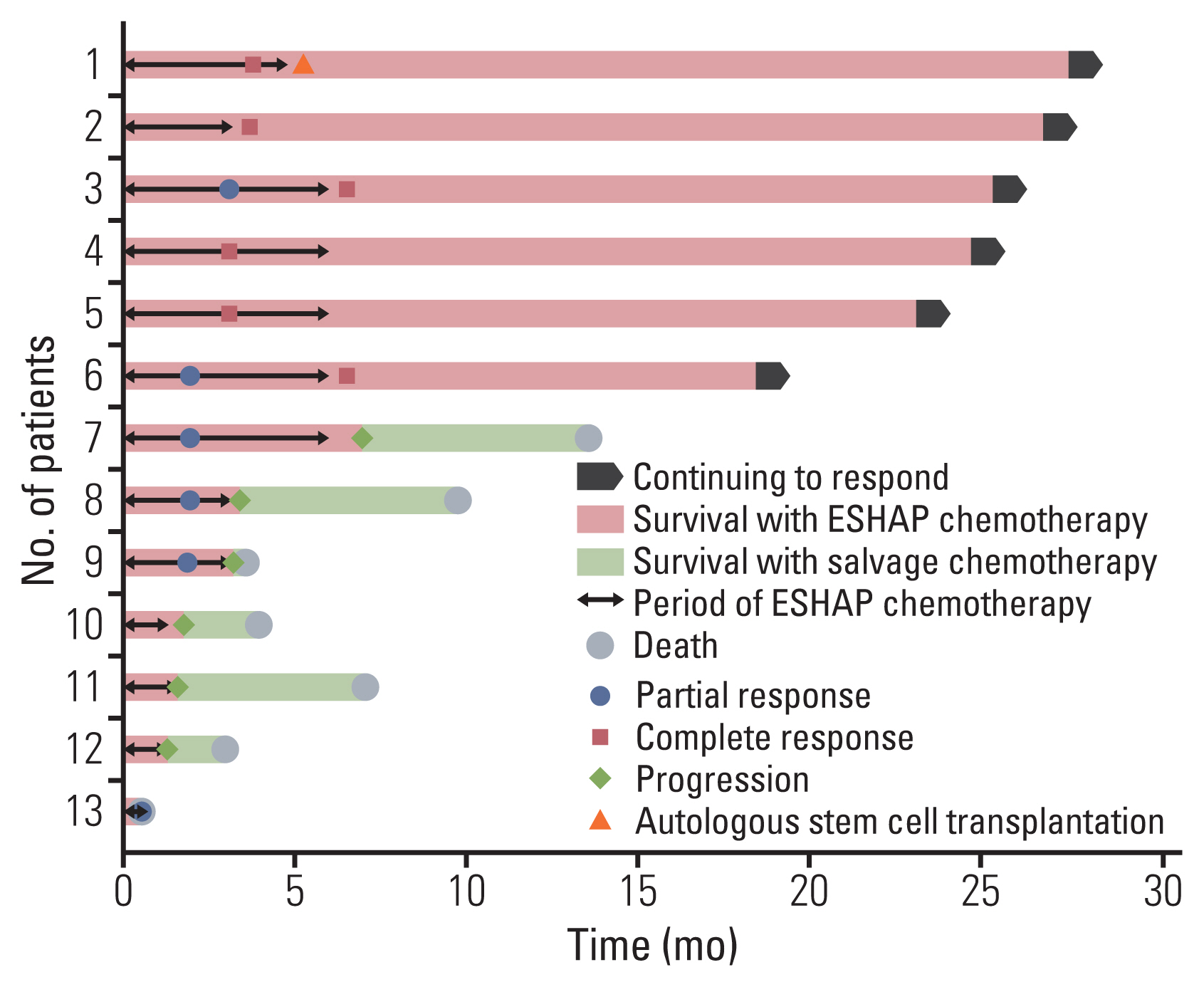
Table 1Characteristics of patients given the roflumilast combined regimen Values are presented as median (range) or number (%). ABC, activated B-cell; B2MG, beta-2 microglobulin; DLBCL, diffuse large B cell lymphoma; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B-cell; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ORR, overall response rate; R-CHOP, rituximab, cyclophosphamide, adriamycin, vincristine, and prednisolone. Table 2Survival and response to the roflumilast combined regimen treatment Table 3Adverse events according to the use of roflumilast combined regimen and their grades References1. Lee H, Park HJ, Park EH, Ju HY, Oh CM, Kong HJ, et al. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res Treat. 2018;50:222–38.
2. Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146–71.
3. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
4. Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1987;316:1493–8.
5. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
6. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
7. National Comprehensive Cancer NetworkNCCN guidelines for diffuse large B-cell lymphoma. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020.
8. Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:81–90.
9. Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, et al. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood. 2005;105:308–16.
10. Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, et al. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. 2016;30:617–26.
11. Bosnjak M, Ristic B, Arsikin K, Mircic A, Suzin-Zivkovic V, Perovic V, et al. Inhibition of mTOR-dependent autophagy sensitizes leukemic cells to cytarabine-induced apoptotic death. PLoS One. 2014;9:e94374.
12. Kim SW, Rai D, Aguiar RC. Gene set enrichment analysis unveils the mechanism for the phosphodiesterase 4B control of glucocorticoid response in B-cell lymphoma. Clin Cancer Res. 2011;17:6723–32.
13. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Primary analysis of juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2017;130(Suppl 1):577.
14. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–65.
15. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–6.
16. Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–84.
17. Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520.
18. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74.
19. Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–37.
20. Zhao S, Dong X, Shen W, Ye Z, Xiang R. Machine learning-based classification of diffuse large B-cell lymphoma patients by eight gene expression profiles. Cancer Med. 2016;5:837–52.
21. Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, et al. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther. 2017;11:1905–15.
22. Galmarini CM, Jordheim L, Dumontet C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev Anticancer Ther. 2003;3:717–28.
23. Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP: an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–76.
24. Ezzat AA, Khalifa F, Berry J, Khan B, Raja MA, Abdel-Warith A. E-SHAP: an effective treatment in selected patients with relapsed non-Hodgkin’s lymphoma. Ann Oncol. 1994;5:453–6.
25. Choi CW, Paek CW, Seo JH, Kim BS, Shin SW, Kim YH, et al. ESHAP salvage therapy for relapsed or refractory non-Hodgkin’s lymphoma. J Korean Med Sci. 2002;17:621–4.
26. Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–36.
27. Kim DU, Kwak B, Kim SW. Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer. Biochem Biophys Res Commun. 2019;508:825–31.
28. Domvri K, Zarogoulidis K, Zogas N, Zarogoulidis P, Petanidis S, Porpodis K, et al. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J Cancer. 2017;8:3648–56.
|
|
||||||||||||||||||||||||||||||||||||||||||||