AbstractPurposeThis study aimed to evaluate the effect of waiting time, from diagnosis to initiation of definitive concurrent chemoradiation (CCRT), on overall survival in cervical cancer patients.
Materials and MethodsPatients with cervical cancer who were treated with definitive CCRT between 2000 and 2017 were retrospectively reviewed. Time from initial pathological diagnosis to definitive CCRT was analyzed both as a continuous variable (per day) and as a categorical variable in two groups (group 1 ≤ median, group 2 > median). Patients with a waiting time of more than 60 days were excluded.
ResultsThe median waiting time was 14 days (0–60). There were differences between group 1 and group 2 in age and chemotherapy regimens. However, no significant difference was found in the International Federation of Gynecology and Obstetrics stage, cell type, or the number of cycles of chemotherapy received during CCRT. A longer waiting time was associated with poorer overall survival on the Kaplan-Meier curve (group 1 vs. group 2, p=0.042). On multivariate analysis, intervals as either a continuous variable (hazard ratio [HR], 1.023; 95% confidence interval [CI], 1.006 to 1.040; p=0.007) or a categorical variable (HR, 1.513; 95% CI, 1.073 to 2.134; p=0.018), FIGO stage, cell type, and the number of cycles of chemotherapy received during CCRT were significant independent prognostic factors for overall survival.
IntroductionApproximately 570,000 cases of cervical cancer and 311,000 deaths from the disease occurred in 2018 worldwide. This was the fourth most common cancer in women suggesting it remains a major public health problem on a global scale [1]. Disease advancement and mortality were mainly attributed to the primary treatment of radiation alone. Concomitant platinum-based chemotherapy during radiation was proven to have superior oncological outcomes to radiation alone. Since 1999, the standard treatment for patients with advanced cervical cancer has been concurrent chemoradiation (CCRT) [2–6].
In general, a prompt diagnosis followed by immediate treatment is advocated for treating cancers. Radiobiological modeling, illustrates that delays in the initiation of radiotherapy treatment have an adverse effect on tumor control, especially in fast-growing tumors [7]. Nevertheless, patients with cervical cancer often have delayed treatment in reality [8] for various reasons [9]. It is debatable whether a delay between the diagnosis and treatment of cervical cancer has a negative effect on the patient’s clinical outcome [10,11]. One of the possible reasons for these conflicting results is heterogeneity in the definition of waiting time, distributions of International Federation of Gynecology and Obstetrics (FIGO) stage (early, advanced stage, or all stage), cohorts with various waiting time frames, and various treatment modalities (surgery, radiotherapy, or any types of treatment) among the studies.
Most studies of cervical cancer investigating the role of waiting time define late initiation of treatment as being more than 1–2 months from the pathological diagnosis [12]. Based on this, the time spent waiting to begin radiotherapy is considered an indicator of the quality of care [13] within this time frame [14,15]. For example, in 2012, a law that regulates an acceptable waiting time of up to 60 days for beginning the treatment of new cases of cancer was passed in Brazil [16]. However, it remains unknown if the role of waiting time between the pathological diagnosis and the initiation of treatment within this qualified time frame in the era of CCRT for cervical cancer is significant.
The present study was undertaken in order to further explore this issue by assessing the effect of waiting time from diagnosis to treatment initiation on the outcome of patients with cervical cancer who were treated with CCRT within 2 months of the pathological diagnosis.
Materials and MethodsAmong the 460 patients who had radiation-based treatment for newly diagnosed cervical cancer between 2001 and 2017, patients who had radiation alone (n=51), a waiting time of more than 60 days (n=8), and/or were diagnosed with other malignancies (n=12) during the study period were excluded, leaving 389 patients with cervical cancer for the final analysis (S1 Fig). Waiting time was defined as days from initial pathological diagnosis confirmed on pathological report to the first day of CCRT.
Data, including patients’ demographics, cell type, FIGO stage (ver. 8), and the regimen of chemotherapy during CCRT, were collected retrospectively. For locally advanced cervical cancer (IB2 and IIA2 or above), definitive CCRT was recommended unless the patients had medical co-morbidities that were not eligible for receiving cytotoxic agents. For early cervical cancer patients who are not well enough to receive radical surgery, definitive CCRT (which was more preferred), or radiotherapy alone was recommended by the attending physician. For chemotherapy during CCRT, cisplatin-based combination chemotherapy or weekly cisplatin was used based on the attending physicians’ preference. However, recently weekly cisplatin has been used primarily, and details of the chemotherapy in each regimen was described previously [17]. In terms of numbers of cycles of chemotherapy in each regimen, six cycles for a single-agent weekly platinum and four cycles for platinum-based combination chemotherapies were recommended. We defined early discontinuation of chemotherapy as being received equal to, or less than half of cycles initially planned during CCRT (e.g., 1, 2, or 3 cycles for a single-agent weekly platinum and 1 or 2 for a platinum-based combination chemotherapy).
Radiotherapy consisted of external beam radiotherapy to the whole pelvis with a standard fractionation (1.8 Gy/fraction, 5 fractions/wk) to a total dose of 45 to 50.4 Gy, depending on the tumor size. For gross pelvic lymph node nodal metastasis, a sequential boost was added. All patients received intracavitary brachytherapy, consisting of high-dose-rate brachytherapy to a total dose of 24 Gy, delivered in 6 fractions (3 times/wk).
Overall survival (OS) was defined as the date of death or last follow-up. Median with a range was used for non-normal distributions after a normality check with the Shapiro-Wilk test. Frequency distributions among categorical variables were compared using a chi-square test or Fisher exact test. Survival curves were calculated using the Kaplan-Meier method with a log-rank test. The Cox proportional hazards model was used for multivariate analysis. A p < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software ver. 25.0 (IBM Corp., Armonk, NY).
ResultsBasic patients’ characteristics are presented in Table 1. Median waiting time for all patients (n=389) was 14 (0–60) days, and the median age at diagnosis was 55 years (range, 25 to 85 years). On the first and second week after pathological diagnosis, 17.7% (69/389) and 37.5% (146/389) of patients began CCRT. A total of 91.0% (354/389) of patients initiated CCRT within 4 weeks after pathological diagnosis, and only 9.0% (35/389) began CCRT thereafter, as shown in Fig. 1. When the patients were divided into two groups based on the median waiting time, patients who had shorter waiting times (group 1; intervals ≤ 14 days) were younger than those with longer waiting times (group 2; intervals > 14 days) (53 years vs. 57 years, p < 0.001). The two groups did not differ in the FIGO stage, cell type, and/or the number of cycles of chemotherapy received. However, there was a significantly larger percentage of patients with weekly cisplatin in group 2 than in group 1 (64.0% vs. 52.1%, p=0.015). The details of chemotherapeutic regimens were listed in S2 Table. In addition, the duration of CCRT was not significantly different between group 1 and group 2 (56 days vs. 52 days, p=0.166).
Shorter interval was significantly associated with better OSs as shown in Fig. 2. When waiting time was analyzed as a continuous variable (per day), shorter waiting times were also associated with better OS (hazard ratio [HR], 1.019; 95% confidence interval [CI], 1.003 to 1.036; p=0.020) in the univariate analysis. Multivariate analysis revealed that a longer waiting time was an independent poor prognostic factor for OS as a continuous variable (HR, 1.023; 95% CI, 1.006 to 1.040; p=0.007) as shown in Table 2. Besides, an advanced FIGO stage, non-squamous cell type, and early discontinuation of chemotherapy were also independent poor prognostic factors for OS. The same results were found when waiting time was analyzed as a categorical variable (group 1 vs. group 2). In a multivariate analysis showing patients with waiting times more than 14 days showed poorer OS than those with equal to or less than 14 days (HR, 1.513; 95% CI, 1.073 to 2.134; p=0.018) (S3 Table).
Further analyses were performed to investigate the association between intervals and progression-free survival and recurrence patterns (pelvic vs. systemic recurrence) according to intervals. Better recurrence-free survival showed in group 1 when compared with group 2 which is in line with that of OS as shown in S4 Fig. However statistical significance was not found (p=0.129). In terms of recurrence patterns according to interval, local recurrence rates in both groups were similar as 8.4% in group 1 and 13.7% in group 2 (p=0.279) as shown in S5 Table.
DiscussionIn this study, we evaluated the association between waiting time, from the initial pathological diagnosis to initiation of definitive CCRT, and survival of patients with cervical cancer. As shown in Table 3, waiting time in our cohort (median, 14 days; range, 0 to 60 days) was relatively short as compared to those of previously published studies. In our time frame, we found that a shorter waiting time was also associated with better OS regardless of whether it was analyzed as a continuous (per day) or categorical (based on 14 days) variable even after adjusting for confounders including age, FIGO stage, and type of chemotherapy.
There have been several studies that have investigated the waiting time in cervical cancer. Of note, most of the studies showed a longer median waiting time in their study cohorts than this study, from 30 to 114 days showing wide variation among the studies [9,10,16,18,19]. For example, one study showed that among 195 patients between 1990 and 2001, 81% had radiotherapy within 8 weeks of the initial biopsy, and the median waiting time was approximately 6 weeks [11]. Longer waiting times for radiotherapy were found to be associated with diminished survival in this study and, recently, a population-based observational study analyzing 9,693 patients also reported lower OS in patients with a waiting time of more than 90 days until treatment [20], which is consistent with our results. Currently, there has been a consensus regarding the standard limit of waiting times, which is within 1 to 2 months, in various institutions from different countries (e.g., within 4 weeks in Canada [21] or 60 days in Brazil [16]). For example, the Canadian Institute for Health Information established 28 days as the standard time limit for treating cancer with radiotherapy from 2011 onward [21]. A study designed under a special program for enhancing cervical cancer screening, showed a relatively short median waiting time at approximately 3 weeks. In New Zealand, a median of 26 days was reported in cervical cancer patients for radiotherapy [22]. However, the study did not analyze survivals based on different waiting times within their time frame [12]. It remains unclear whether a shorter waiting time is associated with better survival within the recommended time frames, particularly in patients who are candidates for primary CCRT. In our study, 91.0% patients began CCRT within 4 weeks after pathological diagnosis and a longer waiting time as either a continuous or categorical variable was associated with poorer OS. This suggested the initiation of CCRT after pathological diagnosis may be expedited even within the recommended standard time limit for treating cervical cancer with CCRT.
In patients with early cervical cancer treated with surgery, the role of waiting time on survival showed mixed results. For example, 441 patients with FIGO IA2 to IB1 cervical cancer were reviewed to evaluate the impact of surgical waiting time on survival [19]. The median waiting time was 43 days, and longer than 8 weeks in waiting time was associated with poorer OS (HR, 3.4; 95% CI, 1.3 to 9.2; p=0.021) in multivariate analysis. On the contrary, Umezu et al. [10] reviewed the records of 117 patients who underwent surgical resection for stage IA–IIA cervical cancer and found that waiting time to the operation from the initial visit to surgical intervention did not adversely affect the outcome of cervical cancer (within the time frames in which the median was 48 days with a range of 20–92 days). There are several differences between the two studies including definition of the waiting time (date of diagnosis to surgery vs. the first visit to surgery), distribution of FIGO stage (e.g., 89.8% [IB1] vs. 71.8% [IB1]), and the rate of adjuvant CCRT (5.4% vs. 54.7%) which may partially explain the discrepancies in the results between the two studies. Another two studies included all stage cervical cancers with various treatments also showed mixed results. For example, in 2014, 321 cervical cancer patients with all FIGO stages, who had various treatments such as surgery, CCRT, or neoadjuvant chemotherapy were eligible to evaluate the effect of treatment delay on prognosis [18]. On multivariate analysis, the authors found a longer waiting time from diagnosis to treatment was not associated with worse survival. On the other hand, a nation-wide population-based study consisting of 9,081 patients with FIGO I to IV cervical cancer showed that delaying treatment for ≥ 4 months can significantly raise mortality risk in cervical cancer patients [23]. It is interesting to see these mixed results in these studies where early or all stages were included. This was opposed to studies exclusively including advanced stages or patients who had CCRT, which showed consistency in poorer survival from delayed waiting times as described above. As there continues to be limited evidence in early cervical cancer as opposed to early endometrial cancer [24,25] in which a longer wait-time was associated with poorer survival, a further study that is well designed with a large number of patients will be essential in early cervical cancer.
Age is one of the independent factors involved in a longer waiting time in cervical cancer. For example, although a cohort study reported the effectiveness of a screening program in reducing cervical cancer mortality, the positive effects of the screening program were not observed in older women because of treatment delays [26]. In another study, patients of advanced age (≥ 75 years) had 2.42 times the odds of delaying treatment as that of patients ≤ 44 years of age [23] or age older than 40 years (odds ratio, 2.18; 95% CI, 1.26 to 3.77) [9] were positively associated with treatment initiation delay. We also found patients who had waiting times of more than 14 days were significantly older than those with equal to or less than 14 days. In addition to age, it was reported that patients’ medical condition affected waiting time. For example, higher rates of treatment delays were observed among patients who had a severe comorbidity (e.g., Charlson comorbidity index [23]). In our study, we divided all patients into two groups based on the type of regimen (single vs. combination) or the number of cycles of chemotherapy during CCRT (less than a half vs. more than a half of planned doses). Since eligibility criteria for each chemotherapy included the patients’ general condition such as Eastern Cooperative Oncology Group performance score, we assumed that these two factors can be used as surrogate markers representing the patients’ general medical condition and were adjusted via multivariate analysis.
There are other parameters in relation to a delayed waiting time. Besides the need for a medical point of view [18] such as pathologist reviews, request for a second opinion, and ovarian transposition prior to CCRT, other factors including the patients’ socioeconomic status, poor access to services, and hospital setting (e.g., regional vs. tertiary, low volume vs. high volume.) may also have affected waiting times [8,23,26]. Our study is limited by these factors. Another limitation is that a cut-off waiting time, which can be useful at a clinic, was not drawn in our study. However, our study suggests that reducing waiting times for CCRT may be one of the strategies to increase survival in patients with advanced cervical cancer.
Delayed treatment can be categorized into four components—patient delay, healthcare provider delay, referral delay, and system delay [27]. Referral delay may be a major determinant of waiting time. For example, the median of the interval between the diagnostic confirmation and the first oncological consultation at the clinic and between the first oncological consultation and the first radiotherapy session was 33 days and four days, respectively [16]. Furthermore, another study showed that the interval between the first oncological consultation and the effective administration of oncologic treatment remained stable, being less than 10 days throughout the study period [28]. Since referral time is a modifiable factor, it is suggested that decreasing the waiting time is achievable by making the referral system more efficient.
Apart from the retrospective study design, there are other limitations in this study. First, there was heterogeneity in the regimens of chemotherapy during CCRT. A meta-analysis showed that radiotherapy plus platinum-based doublet therapy improved the survival compared to radiotherapy plus platinum single-agent therapy in patients with locally advanced cervical cancer [29], which is not consistent with our results. However, it may be difficult to assume that various chemotherapy methods has significantly affected the results of our study since this was adjusted in the multivariate analysis. Second, pathological parameters, including node assessment and primary tumor size, were not considered in the current study, which might have generated bias in interpreting the results. Third, recurrence-free survival does not seem to be affected by intervals as much as OS. However, the fact that there may be underestimated number of recurrences due to follow-up loss during study period may partly explain failure to detect statistical significance with low sensitivity from lack of power. Despite these limitations, our findings provide physicians and policymakers with some evidence to improve survival rates by decreasing waiting time in patients with locally advanced cervical cancer who are a candidate for primary CCRT.
In conclusion, our study shows the importance of minimizing time delays from diagnosis to treatment, especially for cervical cancer patients who are candidates for primary CCRT. Stakeholders in the medical health system should make an effort to expedite delivering treatments, within the limited medical resources, for patients with cervical cancer.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board (IRB No. 2020-04-126) of our institution, and waived the requirement for obtaining informed consent. Author Contributions Conceived and designed the analysis: Noh KW, Kim B, Lee YY. Collected the data: Noh KW, Kim B, Lee YY. Contributed data or analysis tools: Noh KW, Kim B, Lee YY. Performed the analysis: Noh KW, Kim B, Lee YY. Wrote the paper: Noh KW, Kim B, Lee YY. Supervision: Choi CH, Kim TJ, Cho WK, Park W. Project administration: Lee JW, Kim BG, Bae DS. Validation: Cho WK. AcknowledgmentsThis work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019R1F1A1063567).
This research was supported by Research and Business Development Program through the Korea Institute for Advancement of Technology (KIAT) funded by the Ministry of Trade, Industry and Energy (MOTIE) (P0014051).
We would like to thank Editage (www.editage.co.kr) for English language editing.
Fig. 1Number of patients based on waiting time from pathological diagnosis confirmed on pathologic report to the first day of concurrent chemoradiation. 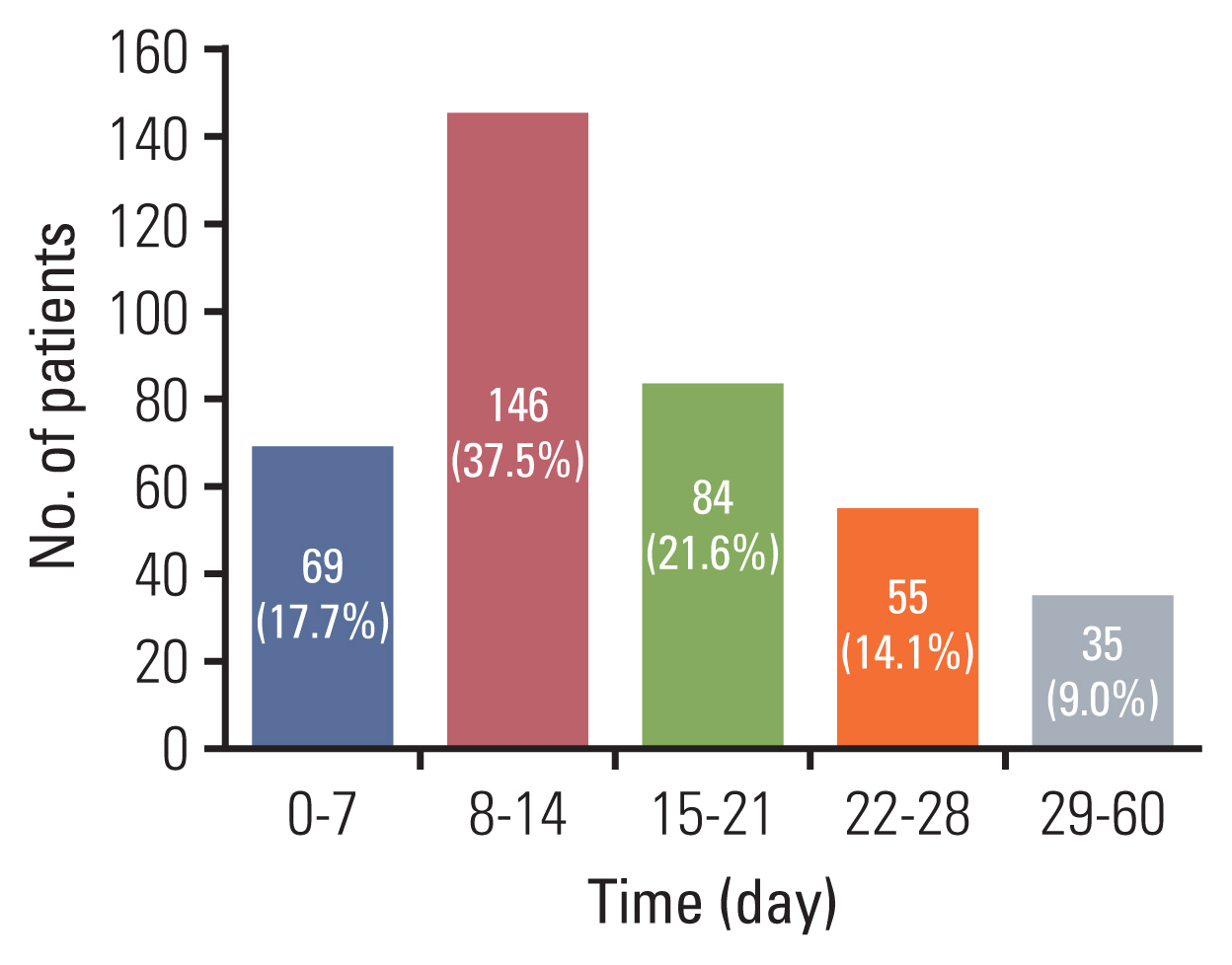
Fig. 2Kaplan-Meier survival curves for overall survival. Patients were divided into four groups per week of intervals (A) or two groups based on median waiting time (B). 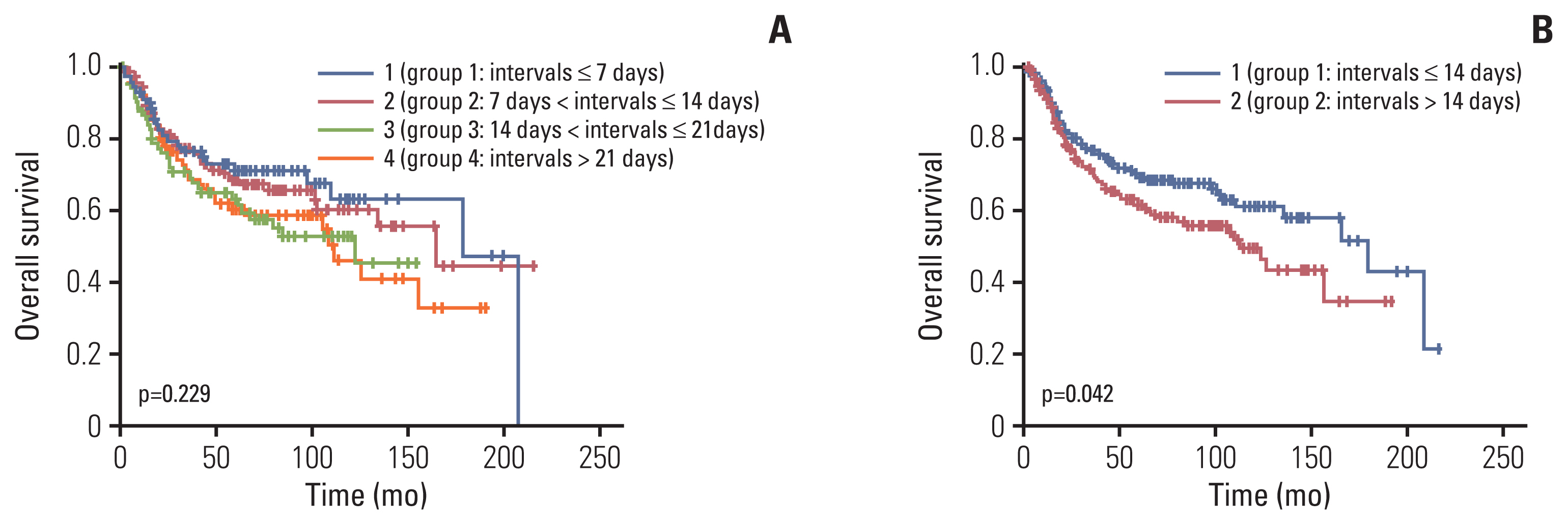
Table 1Patients’ baseline characteristics
Table 2Univariate and multivariate analyses for overall survival
Table 3Literature review of studies investigating waiting time in cervical cancer
AT, additional therapy; Bx, biopsy; CCRT, concurrent chemoradiation; CS, clinical staging; DFS, disease free survival; DSS, disease specific survival; Dx, diagnosis; EUA, examination under anesthesia; FIGO, International Federation of Gynecology and Obstetrics; IT, initial therapy; IV, initial visit; NA, not available; OS, overall survival; RO, radiation oncology consultation; RT, radiotherapy; TE, therapy end time. References1. Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203.
2. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61.
3. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13.
4. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–48.
5. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43.
6. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53.
7. Wyatt RM, Jones BJ, Dale RG. Radiotherapy treatment delays and their influence on tumour control achieved by various fractionation schedules. Br J Radiol. 2008;81:549–63.
8. Mackillop WJ, Zhou Y, Quirt CF. A comparison of delays in the treatment of cancer with radiation in Canada and the United States. Int J Radiat Oncol Biol Phys. 1995;32:531–9.
9. Ferreira da Silva I, Ferreira da Silva I, Koifman RJ. Cervical cancer treatment delays and associated factors in a cohort of women from a developing country. J Glob Oncol. 2019;5:1–11.
10. Umezu T, Shibata K, Kajiyama H, Yamamoto E, Mizuno M, Kikkawa F. Prognostic factors in stage IA–IIA cervical cancer patients treated surgically: does the waiting time to the operation affect survival? Arch Gynecol Obstet. 2012;285:493–7.
11. Choan E, Dahrouge S, Samant R, Mirzaei A, Price J. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int J Radiat Oncol Biol Phys. 2005;61:1071–7.
12. Benard VB, Howe W, Royalty J, Helsel W, Kammerer W, Richardson LC. Timeliness of cervical cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program. J Womens Health (Larchmt). 2012;21:776–82.
13. Choi CH, Ryu JY, Cho YJ, Jeon HK, Choi JJ, Ylaya K, et al. The anti-cancer effects of itraconazole in epithelial ovarian cancer. Sci Rep. 2017;7:6552.
14. Croke J, Fyles A, Barbera L, D’Souza D, Pearcey R, Stuckless T, et al. Radiation therapy quality-of-care indicators for locally advanced cervical cancer: a consensus guideline. Pract Radiat Oncol. 2016;6:315–23.
15. Cionini L, Gardani G, Gabriele P, Magri S, Morosini PL, Rosi A, et al. Quality indicators in radiotherapy. Radiother Oncol. 2007;82:191–200.
16. Nascimento MI, Silva GA. Waiting time for radiotherapy in women with cervical cancer. Rev Saude Publica. 2015;49:92.
17. Lee YY, Park W, Huh SJ, Yoon A, Park JY, Choi CH, et al. Platinum-based combination chemotherapy vs. weekly cisplatin during adjuvant CCRT in early cervical cancer with pelvic LN metastasis. Anticancer Res. 2013;33:4675–81.
18. Perri T, Issakov G, Ben-Baruch G, Felder S, Beiner ME, Helpman L, et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24:1326–32.
19. Nanthamongkolkul K, Hanprasertpong J. Longer waiting times for early stage cervical cancer patients undergoing radical hysterectomy are associated with diminished long-term overall survival. J Gynecol Oncol. 2015;26:262–9.
20. Chen CP, Kung PT, Wang YH, Tsai WC. Effect of time interval from diagnosis to treatment for cervical cancer on survival: a nationwide cohort study. PLoS One. 2019;14:e0221946.
21. Paik ES, Shim M, Choi HJ, Lee YY, Kim TJ, Lee JW, et al. Impact of lymphadenectomy on survival after recurrence in patients with advanced ovarian cancer without suspected lymph node metastasis. Gynecol Oncol. 2016;143:252–7.
22. Capelle L, Stevens W, Brooks S. Management pathway for patients with cervical cancer in the Auckland region 2003–2007. J Med Imaging Radiat Oncol. 2011;55:337–43.
23. Shen SC, Hung YC, Kung PT, Yang WH, Wang YH, Tsai WC. Factors involved in the delay of treatment initiation for cervical cancer patients: a nationwide population-based study. Medicine (Baltimore). 2016;95:e4568.
24. Strohl AE, Feinglass JM, Shahabi S, Simon MA. Surgical wait time: a new health indicator in women with endometrial cancer. Gynecol Oncol. 2016;141:511–5.
25. Elit LM, O’Leary EM, Pond GR, Seow HY. Impact of wait times on survival for women with uterine cancer. J Clin Oncol. 2014;32:27–33.
26. Gyenwali D, Khanal G, Paudel R, Amatya A, Pariyar J, Onta SR. Estimates of delays in diagnosis of cervical cancer in Nepal. BMC Womens Health. 2014;14:29.
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: cancer. Br J Cancer. 2005;92:1959–70.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||