AbstractPurposeThis study aimed to investigate the clinical outcomes with gemcitabine-carboplatin (GCb), the standard treatment for patients with advanced urothelial carcinoma (UC) who are ineligible for cisplatin-based regimens, in advanced UC patients with a glomerular filtration rate (GFR) < 30 mL/min.
Materials and MethodsA retrospective cohort study involving GCb-treated advanced UC patients with GFR < 60 mL/min (n=89) was performed. Clinical outcomes were compared between subgroups with GFR < 30 mL/min and GFR ≥ 30 mL/min but < 60 mL/min.
ResultsMost baseline characteristics were comparable between the two subgroups. Patients with GFR < 30 mL/min had a significantly lower objective response rate (12.5%) compared to those with higher GFR levels (56.7%) (p=0.004). The number of GCb cycles was significantly lower in patients with GFR < 30 mL/min (median 2 cycles) than in those with higher GFR levels (median 6 cycles) (p=0.002). Compared to those with GFR ≥ 30 mL/min but < 60 mL/min, patients with GFR < 30 mL/min showed significantly worse progression-free survival (PFS) and overall survival (OS) (p < 0.001 for both). Further stratification of patient subgroups according to their GFR (i.e., GFR ≥ 45 mL/min but < 60 mL/min vs. GFR ≥ 30 mL/min but < 45 mL/min vs. GFR < 30 mL/min) revealed significantly different PFS and OS (p < 0.001 for both).
IntroductionUrothelial cancer (UC) is a set of tumors originating from the urothelial cells lining the urethra, bladder, ureters and renal pelvis [1]. For patients with locally advanced and unresectable or metastatic UC, cisplatin-based doublet chemotherapy has been the mainstay treatment, leading to a median overall survival (OS) of 14 to 15 months [2–4]. However, a substantial proportion of advanced UC patients are ineligible for cisplatin-based treatment because of obstructive uropathy or other chronic kidney disease (CKD) associated with older age and comorbidities, as well as a poor performance status (PS) [1,5,6]. To overcome this problem, the EORTC 39086 study was conducted for cisplatin-unfit patients and showed that the use of second-generation platinum carboplatin in combination with gemcitabine (GCb) yielded an objective response rate (ORR) of 41.2% and a median OS of 9.3 months with acceptable toxicity profiles [7]. Based on these results, GCb is currently considered a standard front-line therapy for cisplatin-unfit patients.
While cisplatin-ineligibility is generally defined as having a glomerular filtration rate (GFR) < 60 mL/min, Eastern Cooperative Oncology Group (ECOG) PS 2, grade 2 hearing loss or peripheral neuropathy, and/or symptomatic congestive heart failure [5,6], patients who fall into this category make up substantially heterogeneous populations. These patients exhibit a wide range of renal function, spanning CKD stages 3–5. In particular, cancer patients with CKD stage 4–5 (GFR < 30 mL/min) show extremely poor survival outcomes compared to those with a relatively preserved renal function [8]. Therefore, the establishment of optimal therapeutic strategies according to the different GFR categories, especially for GFR < 30 mL/min, are warranted. However, the clinical outcomes of patients with GFR < 30 mL/min who are treated with systemic chemotherapies, including GCb, have not been reported in detail since these patients are generally excluded from studies involving Cb-based chemotherapy. In a recent phase II COACH study, we noticed that none of the three patients with GFR < 30 mL/min showed an objective response to GCb [9]. This finding raises an important question regarding the clinical role of GCb in advanced UC patients with GFR < 30 ml/min, and it requires confirmation in a larger cohort.
In this retrospective cohort study involving advanced UC patients treated with GCb, we aimed to investigate their clinical outcomes according to their different GFR categories.
Materials and Methods1. Study patientsThe study patients were identified from a retrospective cohort of UC patients (n=288) treated with GCb at Asan Medical Center (Seoul, Korea) between April 2011 and August 2020. A total of 89 patients with GFR < 60 mL/min according to the Cockcroft-Gault formula were treated with first-line GCb and were included as the study population, after excluding patients who received GCb as part of neoadjuvant or adjuvant treatment (n=95), those whose therapeutic line of GCb was 2nd line or above (n=56) and those with GFR ≥ 60 mL/min (n=48). Their baseline patient characteristics were retrieved from their electronic medical records, including demographic factors, ECOG PS, the presence of diabetes mellitus and hypertension, the primary tumor site, the number of metastatic sites, the disease setting, previous surgical resection or (neo)adjuvant chemotherapy, the site of metastasis and the hemoglobin level. The use of a contrast-enhanced computed tomography (CT) scan at baseline and at the time of the response evaluation was also examined.
2. Study endpointsThe primary endpoint of this study was to compare the ORR between patient subgroups with different GFRs. The secondary endpoints included progression-free survival (PFS) and OS stratified by different GFR levels.
3. Treatment and follow-upGemcitabine (1,000 mg/m2 intravenously on days 1 and 8) and carboplatin (area under the curve 4.5 intravenously on day 1) were administered every 3 weeks until disease progression or unacceptable adverse events occurred. Study patients were followed-up and examined on the days of chemotherapeutic drug administration (days 1 and 8 of each cycle). Complete blood cell counts and routine chemistry laboratory tests were conducted on the follow-up days. CT scans were performed every 8 to 12 weeks for disease evaluation. When disease progression was clinically suspected during the follow-up, additional CT scans were performed. The Response Evaluation Criteria In Solid Tumors (RECIST) ver. 1.1 was used to grade the tumor responses. Relative dose intensity (RDI) was defined as the ratio of the actually delivered dose intensity of chemotherapy to the standard dose intensity [10]. The RDI of gemcitabine and carboplatin was calculated for the first two cycles of chemotherapy (i.e., cycles 1 and 2).
4. Statistical analysesStatistical analyses were performed using R software ver. 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). The chi-square test or Fisher exact test was used to compare categorical variables among the subgroups. PFS was defined as the time interval from the time of GCb chemotherapy (index date) to the date of disease progression or death. OS was defined as the time interval between the index date and the date of death from any cause. The Kaplan-Meier method was used to estimate survival outcomes, and the log-rank test was used to compare these survival outcomes among the subgroups. Univariate and multivariate analyses of PFS and OS were performed using Cox proportional hazards models. The examined variables included age, sex, disease setting (recurrent disease vs. initially metastatic disease), primary tumor site (bladder primary vs. non-bladder primary), the presence of lymph node and visceral metastasis, and estimated GFR (eGFR) < 30 mL/min. Variables with a potential relationship (p < 0.10) in the univariate analyses were included in the multivariate analysis. A p-value of < 0.05 was considered statistically significant.
Results1. Patient characteristicsThe baseline characteristics of the study patients (n=89) are summarized in Table 1. Their median age was 73 years (range, 45 to 89 years) and 63 (70.8%) were men. The median number of GCb cycles was 4 (range, 1 to 12). There were 34 (38.2%) and 55 (61.8%) patients with initially metastatic disease and recurrent disease after curative surgical resection, respectively. The ECOG PS was 0, 1, and 2 in eight (9.0%), 50 (56.2%), and 31 (34.8%) patients, respectively. The bladder was the primary tumor site in about half of the study population (49.4%). Lymph node and visceral metastases were present in 52 (58.4%) and 48 (53.9%) patients, respectively (Table 1).
We next subdivided the patient subgroups according to their baseline eGFR: patients with CKD stage 3 (≥ 30 mL/min but < 60 mL/min) (n=68) vs. CKD stage 4–5 (< 30 mL/min) (n=21). As shown in Table 1, most baseline characteristics were comparable between the two subgroups, except for the higher proportion of patients having liver metastasis in patients with GFR < 30 mL/min (33.3% vs. 10.3%, p=0.028). The proportion of patients with diabetes mellitus and hypertension tended to be higher in patients with eGFR < 30 mL/min, but the differences were not statistically significant (Table 1). The proportion of patients who underwent contrast-enhanced CT was significantly lower in patients with eGFR < 30 mL/min at baseline and at the time of response evaluation.
2. Treatment profiles of gemcitabine plus carboplatinThe number of GCb cycles was significantly lower in patients with GFR < 30 mL/min (median, 2; interquartile range, 1 to 4) than in those with GFR ≥ 30 mL/min but < 60 mL/min (median, 6; interquartile range, 2 to 6) (p=0.002) (Table 2). The RDI of gemcitabine and carboplatin was significant lower in patients with eGFR < 30 mL/min (median, 75.0% vs. 91.9% for gemcitabine and median, 94.4% vs. 100% for carboplatin, respectively). While there was no overall difference in the reason for treatment discontinuation between the two groups, the leading causes for treatment discontinuation were progressive disease (PD) and completion of treatment for patients with GFR < 30 mL/min and those with GFR ≥ 30 mL/min but < 60 mL/min, respectively (Table 2).
1) Response to gemcitabine plus carboplatin according to GFRThe response evaluation graded by RECIST ver. 1.1 revealed that a complete response and a partial response (PR) were achieved in two (2.9%) and 32 patients (47.1%) in the subgroup with GFR ≥ 30 mL/min but < 60 mL/min, whereas there were only two patients (9.5%) who had a PR in patients with GFR < 30 mL/min (Table 3). PD was the best response in five (7.4%) and five (23.8%) patients with GFR ≥ 30 mL/min but < 60 mL/min and those with GFR < 30 mL/min, respectively.
The response was not evaluable in eight patients (11.8%) in the subgroup with GFR ≥ 30 mL/min but < 60 mL/min (unable to tolerate the treatment in 2, death before disease evaluation in 1 and lost to follow-up for 5) and five patients (23.8%) in the subgroup with GFR < 30 mL/min (unable to tolerate the treatment in 1, refusal of further treatment after the first cycle in 1, death before disease evaluation in 1 and lost to follow-up for 2).
Among patients who had their therapeutic response evaluated, patients in the subgroup with GFR < 30 mL/min had a significantly lower ORR (12.5%) compared to those with higher GFR levels (56.7%) (p=0.004).
2) Survival outcomes according to the GFRIn the overall study patients, the median PFS and OS were 6.64 months (95% confidence interval [CI], 5.42 to 8.22 months) and 10.5 months (95% CI, 8.5 to 14.5 months), respectively (S1A and S1B Fig.).
Compared to those with GFR ≥ 30 mL/min but < 60 mL/min, patients with GFR < 30 mL/min showed a significantly worse PFS (median, 2.6 months vs. 7.4 months; p < 0.001) and OS (median, 6.9 months vs. 13.2 months; p < 0.001) (Fig. 1A and B). Patients with GFR < 30 mL/min still showed a worse PFS and OS compared to the patient subgroup with ECOG PS 2 and GFR ≥ 30 mL/min but < 60 mL/min (p=0.031 and p=0.047, respectively) (S2A and S2B Fig.). Multivariate analysis revealed that GFR < 30 mL/min was independently associated with a poor PFS (hazard ratio [HR], 2.57; 95% CI, 1.42 to 4.65; p < 0.001) and OS (HR, 2.94; 95% CI, 1.57 to 5.54; p < 0.001) (S3 Table).
Finally, when the study patients were further stratified according to their different GFR levels (i.e., GFR ≥ 45 mL/min but < 60 mL/min vs. GFR ≥ 30 mL/min but < 45 mL/min vs. GFR < 30 mL/min), the PFS and OS were significantly different among these subgroups (p < 0.001 and p < 0.001, respectively) (Fig. 2A and B).
DiscussionIn this retrospective cohort study, we assessed the impact of different GFR levels on the therapeutic efficacy of GCb in advanced UC patients treated with GCb. We found that advanced UC patients with GFR < 30 mL/min had a significantly lower ORR as well as a worse PFS and OS compared to those with GFR ≥ 30 mL/min but < 60 mL/min. To our knowledge, this is the first study to focus on the clinical outcomes of GCb in advanced UC patients with GFR < 30 mL/min. Our results highlight the importance of considering the GFR in the treatment decision and the need for alternative therapeutic options for advanced UC patients with GFR < 30 mL/min. Since a substantial proportion of advanced UC patients are complicated by obstructive uropathy or renal function impairment accompanied by various comorbidities of the elderly, our data provide practical insights into the use of chemotherapeutic agents for patients with advanced UC.
One of the most notable aspects of our study is the distinctly low ORR (12.5% among those with evaluable disease) with GCb among patients with GFR < 30 mL/min. This value is far lower than the reported ORRs of 41.2% and 48.7% in previous studies of GCb in cisplatin-unfit patients [7,9]. While the exact mechanism of this finding remains to be further studied, a lower ORR might be attributable to a suboptimal dose of carboplatin, which is determined on the basis of the GFR [11]. Indeed, despite the fact that carboplatin is excreted mainly by glomerular filtration [11], the pharamacokinetic profiles of carboplatin in patients with GFR < 30 ml/min remain unknown. Therefore, alternative carboplatin dosing, such as body surface area-based strategies [12], may be considered in this GFR context. In addition, the number of GCb cycles and the RDI of gemcitabine and carboplatin were significantly lower in this subgroup. These results imply that the general medical condition of patients with eGFR < 30 mL/min was not good enough to receive standard doses of GCb. We assume that the significantly lower number of administered cycles and reduced dose intensity of GCb in patients with eGFR < 30 mL/min might have resulted in the lower ORR and a worse PFS in these patients.
In addition to the low response rate, the extremely short PFS (median, 2.6 months) and OS (median, 6.9 months) suggests that GCb confers minimal clinical benefit in patients with GFR < 30 mL/min. These PFS and OS values are numerically much shorter compared to a previously reported PFS of 5 to 6 months and an OS of 9 to 10 months with cisplatin-unfit UC patients treated with GCb [7,9]. In our analysis, patients with GFR < 30 mL/min showed a worse PFS and OS compared to those with ECOG PS 2 in the subgroup with GFR ≥ 30 mL/min but < 60 mL/min. These results suggest that advanced UC patients with GFR < 30 mL/min may be considered a distinct entity among cisplatin-unfit patients. In addition, a comparison of survival outcomes among the subgroups with three different GFR categories (i.e., GFR ≥ 45 mL/min but < 60 mL/min vs. GFR ≥ 30 mL/min but < 45 mL/min vs. GFR < 30 mL/min) revealed gradually worsening clinical outcomes as the GFR level declined. This finding also points to the critical impact of GFR levels on GCb-treated patients with advanced UC.
Apart from impacting the therapeutic efficacy of systemic chemotherapeutic agents, a low GFR per se can be a strong negative prognosticator in cancer patients [8,13,14]. Reportedly, the median OS is numerically shorter with carboplatin-based regimens in cisplatin-unfit patients (9 to 10 months) [7,9] compared to that of cisplatin-treated patients (14 to 15 months) [2–4]. Therefore, our data showing a worse OS in patients with GFR < 30 mL/min may be due in part to the medical vulnerability of these patients. Nevertheless, our results showing a significantly worse ORR and PFS in patients with GFR < 30 mL/min suggests that the therapeutic efficacy of GCb is suboptimal. There might be an improvement with other chemotherapeutic regimens. Taken together, these results should discourage the use of GCb in advanced UC patients with GFR < 30 mL/min, and they strongly suggest the need for establishing alternative treatment approaches with a better efficacy for these patients.
Immune checkpoint inhibitors (ICIs) may be considered an alternative option in this clinical setting, given their tolerable safety profiles without the need for dose adjustment for kidney impairment [15]. The survival benefit of pembrolizumab shown in the second-line setting also supports the use of ICI in advanced UC patients [16,17]. However, since patients with GFR < 30 mL/min were excluded in recent ICI trials of advanced UC [16–20], the therapeutic efficacy of ICI remains unknown in this GFR context. Furthermore, ICI agents alone failed to show a survival benefit or non-inferiority in terms of therapeutic efficacy in the first-line setting [18–20], which questions the use of these ICI agents alone in this clinical setting.
In a recent phase II study comparing the efficacy of GCb and gemcitabine plus oxaliplatin (GEMOX) in cisplatin-unfit advanced UC patients, we showed that GEMOX might be a feasible option for cisplatin-unfit advanced UC patients [9]. In that study, whereas an objective response was not observed with GCb in the subgroup with GFR < 30 mL/min, three out of five patients (60%) with the same GFR category showed a response to GEMOX, which was well tolerated [9]. Therefore, GEMOX may be one of the feasible options for advanced UC patients with GFR < 30 mL/min, and it deserves further investigation in future studies, either on its own or in combination with ICIs.
The retrospective nature of our study and its relatively small number of patients may limit the interpretation and generalizability of our data. In addition, the fact that the proportion of patients who underwent enhanced CT was significantly lower in patients with eGFR < 30 mL/min suggests that assessment of the presence and extent of disease as well as the response to treatment may have been suboptimal. Nevertheless, our results raise a very important clinical question regarding whether GCb should be used in patients with GFR < 30 mL/min. Given the limited reports on the outcomes of GCb in patients with GFR < 30 mL/min, our results may be used to design future studies and guide clinical decisions.
In conclusion, GCb shows extremely poor clinical outcomes in advanced UC patients with GFR < 30 mL/min. The use of this regimen is discouraged in this population. The establishment of alternative treatment approaches such as GEMOX or ICI is warranted for these patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-0262). Informed consent from the patients was waived. Author Contributions Conceived and designed the analysis: Kim HD, Im HS, Lee JL. Collected the data: Kim HD, Im HS, Lee JL. Contributed data or analysis tools: Kim HD, Im HS, Kim JH, Jeong H, Yoon SK, Park I, Lee JL. Performed the analysis: Kim HD, Kim JH, Jeong H, Yoon SK, Park I, Lee JL. Wrote the paper: Kim HD, Lee JL. Conflict of interest Lee JL reports grants, personal fees and other from Pfizer Korea, grants, personal fees and other from Ipsen Korea, personal fees and other from Sanofi Aventis, personal fees from Novartis Korea, personal fees and other from Astellas Korea, grants, personal fees and other from BMS, grants, personal fees and other from MSD, other from Myovant Science, grants and other from Merck, grants and other from Esai, other from Amgen, grants from Roche, grants and personal fees from AstraZeneca, outside the submitted work. All remaining authors have declared no conflicts of interest. Fig. 1Survival outcomes of patients with estimated glomerular filtration rate (eGFR) < 30 mL/min and those with eGFR ≥ 30 mL/min but < 60 mL/min. Comparison of progression-free survival (A) and overall survival (B) between patients with eGFR < 30 mL/min and those with eGFR ≥ 30 mL/min but < 60 mL/min. 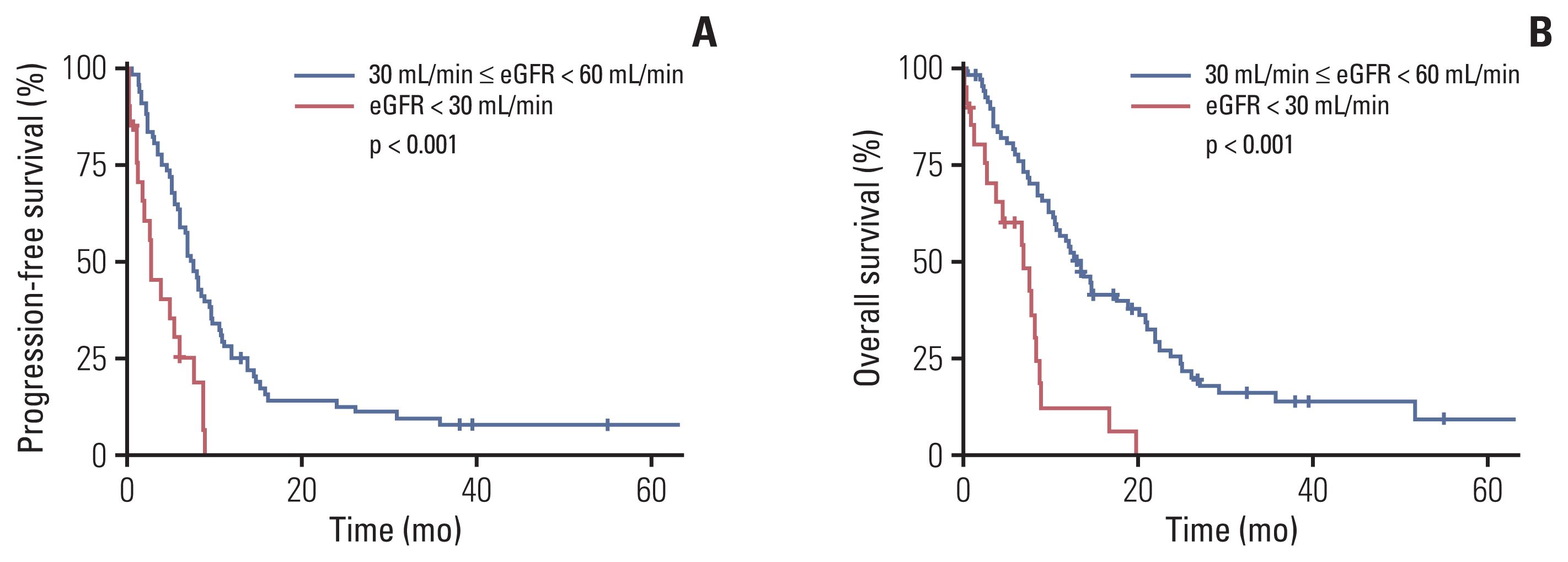
Fig. 2Survival outcomes of patients with estimated glomerular filtration rate (eGFR) < 30 mL/min, eGFR ≥ 30 mL/min but < 45 mL/min, and eGFR ≥ 45 mL/min but < 60 mL/min. Comparison of progression-free survival (A) and overall survival (B) between patient subgroups with eGFR < 30 mL/min, eGFR ≥ 30 mL/min but < 45 mL/min, and eGFR ≥ 45 mL/min but < 60 mL/min. 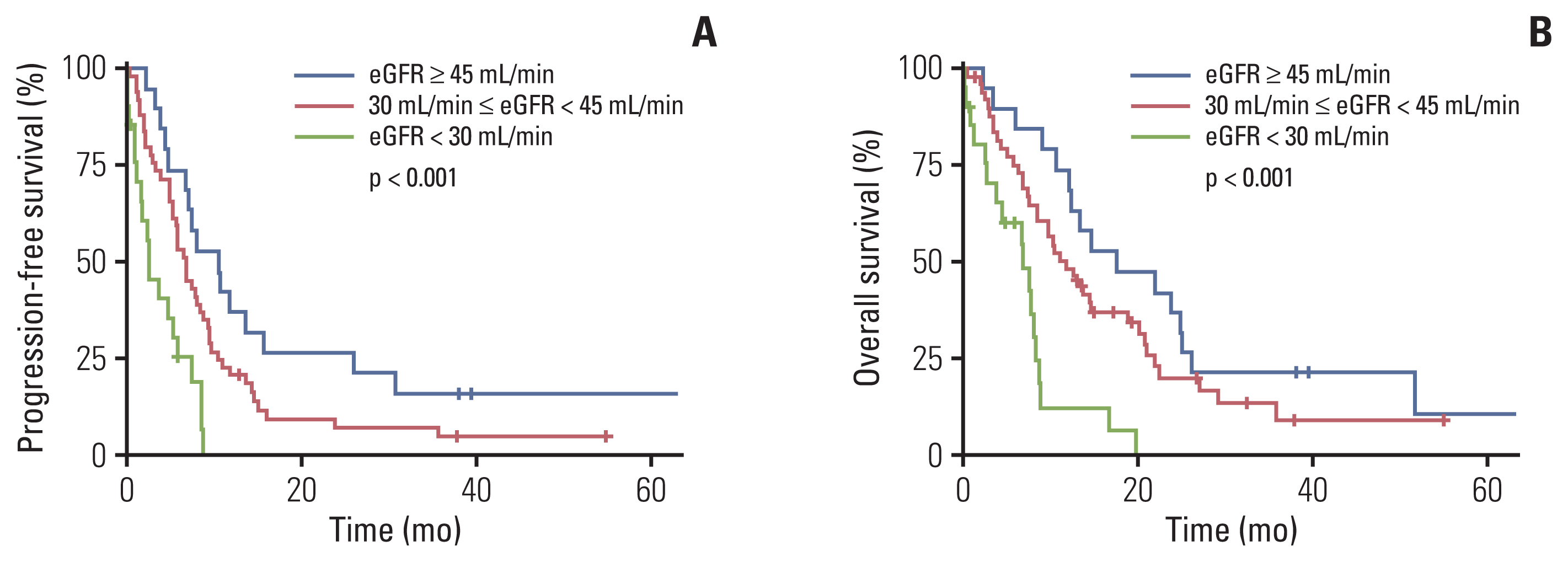
Table 1Clinical characteristics of the study patients
Table 2Treatment profiles of gemcitabine plus carboplatin cycle according to eGFR
Table 3Response to gemcitabine plus carboplatin therapy according to eGFR
References1. Koufopoulou M, Miranda PA, Kazmierska P, Deshpande S, Gaitonde P. Clinical evidence for the first-line treatment of advanced urothelial carcinoma: current paradigms and emerging treatment options. Cancer Treat Rev. 2020;89:102072.
2. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77.
3. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
4. Bellmunt J, Eigl BJ, Senkus E, Loriot Y, Twardowski P, Castellano D, et al. Borealis-1: a randomized, first-line, placebo-controlled, phase II study evaluating apatorsen and chemotherapy for patients with advanced urothelial cancer. Ann Oncol. 2017;28:2481–8.
5. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8.
6. Bukhari N, Al-Shamsi HO, Azam F. Update on the treatment of metastatic urothelial carcinoma. ScientificWorldJournal. 2018;2018:5682078.
7. De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9.
8. Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121–30.
9. Park I, Kim BS, Lim HY, Kim HJ, Lee HJ, Choi YJ, et al. Gemcitabine plus carboplatin versus gemcitabine plus oxaliplatin in cisplatin-unfit patients with advanced urothelial carcinoma: a randomised phase II study (COACH, KCSG GU10-16). Eur J Cancer. 2020;127:183–90.
10. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203–10.
11. Beumer JH, Inker LA, Levey AS. Improving carboplatin dosing based on estimated GFR. Am J Kidney Dis. 2018;71:163–5.
12. Merchan JR, Jhaveri KD. Chemotherapy nephrotoxicity and dose modification in patients with kidney impairment: conventional cytotoxic agents [Internet]. Waltham, MA: UpToDate; 2020. [cited 2020 Dec 20]. Available from: https://www.uptodate.com/contents/chemotherapy-nephrotoxicity-and-dose-modification-in-patients-with-kidney-impairment-conventional-cytotoxic-agents
13. Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer treatments. ESMO Open. 2016;1:e000091.
14. Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis. 2014;63:23–30.
15. Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020;97:62–74.
16. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–26.
17. Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30:970–6.
18. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1574–88.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||