AbstractPurposeAngiosarcoma is a highly aggressive mesenchymal tumor. Although systemic chemotherapy is often considered for the inoperable or metastatic angiosarcoma, the outcome of such treatment is unsatisfactory and poorly delineated.
Materials and MethodsWe reviewed electronic medical records of 75 patients with angiosarcoma who were treated with systemic chemotherapy for inoperable or metastatic disease. Patients were classified as having liver involvement if they had either primary or metastatic hepatic lesions.
ResultsAmong the patients evaluated, 51 patients (68%) were male and 24 patients (32%) had primary cutaneous angiosarcoma. Liver involvement was present in 28 patients (37.3%). A total of 59 patients received first-line weekly paclitaxel (wPac) and showed an objective response rate (ORR) of 23.7% (n=14), a median progression-free survival (mPFS) of 4.0 months (95% confidence interval [CI], 3.0 to 6.1), and a median overall survival (mOS) of 10.2 months (95% CI, 7.0 to 14.6). Among patients without liver involvement, patients receiving wPac (n=35) had significantly prolonged mPFS (5.8 months vs. 3.2 months, respectively; p=0.014) with a tendency for prolonged mOS (13.8 months vs. 11.6 months, respectively; p=0.13) than those receiving other regimens (n=12). A total of 24 patients received second- or later-line pazopanib monotherapy and showed an ORR of 16.7% (n=4), a mPFS of 2.4 months (95% CI, 1.8 to 4.3) and a mOS of 5.4 months (95% CI, 3.5 to not available).
IntroductionAngiosarcoma is a rare and highly aggressive sarcoma subtype and occurs throughout the body at any age [1]. The prognosis of angiosarcoma is generally poor even in localized disease with 5-year overall survival (OS) up to only 60% with a median survival of around 3–10 months for metastatic disease [1–4].
In case of localized disease, standard treatment is surgical resection, sometimes combined with preoperative and postoperative radiation. However, negative surgical margins could be often difficult to achieve especially in the head and neck or in the presence of multifocal disease. These inoperable or metastatic diseases are treated with systemic chemotherapy [5]. Doxorubicin and weekly paclitaxel (wPac) are currently regarded as a preferred option for first- or second-line therapy with a median OS of approximately 8 to 10 months [6,7]. However due to the rarity of the disease, there are only limited efficacy data for such therapies.
Meanwhile, several components involved in angiogenesis have been investigated as potential targets to treat angiosarcoma [8]. These include vascular endothelial growth factor (VEGF) and multiple VEGF receptors, which are the key regulators of angiogenesis that are overexpressed in angiosarcoma [9,10]. Bevacizumab is a recombinant human antibody against VEGF and has been tested in clinical trials both as a monotherapy and in combination with other drugs [11]. Multi-target tyrosine kinase inhibitors (sunitinib, sorafenib, and pazopanib) have also been applied, but the efficacy of such treatment was inconclusive as the numbers of angiosarcoma patients involved were low [1,12].
As consensus treatment strategy for angiosarcoma is not well established, it is necessary to better understand the clinicopathological features of angiosarcoma and correlate them with the treatment response and clinical outcomes. In this retrospective study, we reviewed the patients with advanced angiosarcoma who undergone systemic chemotherapy and evaluated clinical outcomes and their prognostic significance. In addition, we tried to delineate the efficacy of systemic therapies currently available for advanced angiosarcoma.
Materials and Methods1. PatientsWe retrospectively reviewed data of 96 adult patients (age > 18 years) with metastatic or advanced angiosarcoma from 2005 to 2018 at Seoul National University Hospital and Seoul National University Bundang Hospital. The diagnosis of metastatic or advanced angiosarcoma was based on the histopathological analysis according to 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone [13]. Exclusion criteria were: patients who had mixed histology with other soft tissue sarcoma (STS) subtypes (five patients); those who did not receive systemic chemotherapy in locally advanced or metastatic setting (six patients); and those who had no available treatment administration record (10 patients). Patient records were accessed for age, sex, date of last follow-up or deaths, disease site (primary or metastatic), Eastern Cooperative Oncology Group performance status scale (ECOG PS), prior therapy (surgery, radiation, or chemotherapy), and treatment outcome. Tumor grade was evaluated using Federation Nationale des Centres de Lutte le Cancer (FNCLCC) grade.
2. Data collectionWe assessed tumor responses according to Response Evaluation Criteria in Solid Tumors guideline, ver. 1.1 [14]. Objective response rate (ORR) was defined as percentage of patients who experienced partial response (PR) or complete response (CR). The grade of adverse events was assigned according to the National Cancer Institute Common Terminology Criteria for Adverse Events ver. 5.0 [15].
3. Data analysisThe endpoint for the prognostic factor analysis was OS, which was defined as the time from date of treatment initiation to date of death or last contact. The impact of covariates on OS was estimated using Cox models (hazard ratio [HR], 95% confidence interval [CI]). The factors analyzed for univariate analysis were age, sex, ECOG PS, primary sites, presence of liver involvement, lung involvement, bone involvement, lymph node involvement, FNCLCC grade, previous operation intent, pre-chemotherapy laboratory results of albumin and bilirubin. For multivariate analysis, the factors that were significant in univariate analyses were used. Progression-free survival (PFS) was defined as time to progression or death from the initiation of chemotherapy. Kaplan-Meier estimates were used for both OS and PFS analysis. Fisher’s exact test was used to compare ORR. Logistic regression analysis was performed to evaluate the association of factors with response. Continues variables are shown as medians and ranges and categorical variables as percentages. Wilcoxon rank-sum test was used to compare continuous variables. All p-values were two-sided, with p < 0.05 indicating statistical significance. R ver. 3.6.1 software (R Development Core Team, https://www.r-project.org/) was used for statistical analyses.
Results1. Patient demographicsA total of 75 patients who received palliative chemotherapy for advanced angiosarcoma were enrolled. Among these, 24 had primary cutaneous angiosarcoma. The most common primary sites were the scalp (n=19, 25.3%), liver (n=17, 22.7%), and bone (n=7, 9.3%). Two patients had radiation-induced angiosarcoma of the cervix and nasopharynx, respectively, both of whom were involved in previous radiotherapy more than 10 years ago. Other demographic features are summarized in Table 1.
At the time of the first-line palliative chemotherapy, liver involvement either by primary mass or metastatic mass was present in 28 patients (37.3%). Lung, lymph node, and bone involvement were present in 24 (32%), 24 (32%), and 26 (34.7%) of patients, respectively.
2. Clinical outcomes and prognostic factorsThe median OS of the 75 patients from the first-line palliative chemotherapy initiation was 10.2 months (95% CI, 8.6 to 13.8) (Fig. 1A). OS did not differ between patients with primary cutaneous and non-cutaneous angiosarcoma (8.7 months; 95% CI, 6.6 to 21.6 vs. 10.3 months; 95% CI, 7.2 to 14.5; p=0.73) (S1A Fig.). None of primary scalp, primary pulmonary, primary cardiac angiosarcoma, and FNCLCC grade were significantly associated with OS in our analysis (Table 2, S1B Fig.).
Patients with primary hepatic angiosarcoma tended to have poorer OS than those without liver involvement (4.0 months; 95% CI, 2.7 to not available [NA] vs. 12.5 months; 95% CI, 9.6 to 16.7; p=0.05). Patients with liver metastasis that originated from other organs tended to have poorer OS than those without liver involvement (8.7 months; 95% CI, 6.4 to NA for patients with liver metastasis; p=0.06) (Fig. 1B). When patients with primary and metastatic sites of liver were combined as having liver involvement, presence of liver involvement was significantly associated with poor OS (7.0 months; 95% CI, 4.0 to 10.3 vs. 12.5 months; 95% CI, 9.6 to 16.7; p=0.02) (Fig. 1C).
In the univariate Cox proportional hazard regression analysis, ECOG PS and presence of liver involvement were significantly associated with poor OS (HR, 2.54; 95% CI, 1.26 to 5.10; p=0.009 for ECOG PS; HR, 1.96; 95% CI, 1.12 to 3.43; p=0.018 for presence of liver involvement) (Table 2). In addition, higher pre-chemotherapy bilirubin and lower pre-chemotherapy albumin were associated with poor OS (HR, 1.37; 95% CI, 1.14 to 1.63; p < 0.001 for bilirubin; and HR, 0.58; 95% CI, 0.40 to 0.87; p=0.007 for albumin, respectively) (Table 2). In the multivariate Cox proportional hazard regression analysis, presence of liver involvement and pre-chemotherapy bilirubin level were associated with poor OS (HR, 2.27; 95% CI, 1.13 to 4.57; p=0.022 for presence of liver involvement; and HR, 1.27; 95% CI, 1.04 to 1.53; p=0.017 for bilirubin, respectively) (Table 2).
3. First-line weekly paclitaxelA total of 59 patients received first-line wPac. ORR was 23.7% (n=14) including one CR and 13 PR patients (S2 Table 1). ORR in patients with liver involvement was 12.5% (3 of 24 patients) and ORR in patients without liver involvement was 31.4% (11 of 24 patients, p=0.12 compared to patients with liver involvements). Median PFS was 4.0 months (95% CI, 3.0 to 6.1) and OS was 10.2 months (95% CI, 7.0 to 14.6). Response to wPac was associated with prolonged PFS and OS (PFS: 7.0 months; 95% CI, 6.1 to NA for responders vs. 3.0 months; 95% CI, 2.5 to 4.0 for non-responders; p=0.018, Fig. 2A; OS: 21.6 months; 95% CI, 13.8 to NA for responders vs. 7.0 months; 95% CI, 4.0 to 12.1 for non-responders; p=0.012) (Fig. 2B). An example case of a patient who showed favorable response to wPac is shown in S3 Fig. The patient had recurred scalp angiosarcoma with lymph nodes and right parotid gland metastasis which showed complete remission with wPac and PFS of 33.4 months.
ECOG PS, liver involvement, high pre-chemotherapy bilirubin and low pre-chemotherapy albumin level were associated with poor PFS in univariate Cox proportional hazard regression analysis (S4 Table). In multivariate analysis, only pre-chemotherapy albumin level was significantly associated with PFS (HR, 0.56; 95% CI, 0.32 to 0.96; p=0.036). None of the factors were associated with response to first-line wPac in logistic regression analysis (S4 Table). For the adverse events of interest, neuropathy of any grade was observed in 39.0% (n=23) of patients, and most of them (n=19) were tolerable as grade 1. Febrile neutropenia of any grade was observed in 10.2% (n=6) of patients and one patient died of septic shock.
Remaining 16 patients received either one of doxorubicin-based chemotherapy (n=7) or non-doxorubicin-based chemotherapy (n=9) other than wPac as first-line treatment (S5 Table). ORR in these patients was 25% (n=4) including one CR and three PR patients (S2 Table). Compared with these 16 patients, patients who received first-line wPac did not show significant PFS and OS differences (S6 Fig.). However, when patients with liver involvement were excluded, those who received wPac (n=35) showed significantly prolonged PFS (5.8 months; 95% CI, 3.2 to 7.9 vs. 3.15 months; 95% CI, 2.0 to NA, respectively; p=0.014) (Fig. 2C) and tendency to prolonged OS (13.8 months; 95% CI, 10.0 to NA vs. 11.6 months; 95% CI, 9.4 to NA, respectively; p=0.13) (Fig. 2D) compared with patients who received other regimens (n=12).
4. Later-line pazopanibA total of 24 patients received pazopanib monotherapy for advanced angiosarcoma. Most of these patients received pazopanib as second-line treatment (n=18, 75%), and none of the patients received pazopanib as first-line treatment. ORR was 16.7% (n=4) including four PR patients. The median PFS was 2.4 months (95% CI, 1.8 to 4.3 months) (Fig. 3A) and OS was 5.4 months (95% CI, 3.5 to NA months) (Fig. 3B). Responders showed significantly prolonged PFS compared to non-responders (6.4 months; 95% CI, 3.5 to NA vs. 1.9 months; 95% CI, 1.6 to 3.2, respectively; p=0.02) (Fig. 3A) while OS did not significantly differ between responders and non-responders (9.5 months; 95% CI, 3.5 to NA vs. 5.0 months; 95% CI, 3.5 to NA; p=0.58) (Fig. 3B). The most common side effect was hypertension (n=4, 16.7%), followed by diarrhea (n=3, 12.5%) and hand-foot syndrome (n=2, 8.3%).
DiscussionHere we described the clinical outcomes and prognostic factors of advanced angiosarcoma patients treated with systemic chemotherapy. ECOG PS was a significant prognostic factor in our study, which is consistent with previous literature [4]. Primary hepatic angiosarcoma had been associated with poor prognosis [4], and we found that liver involvement by primary or metastatic mass was associated with poor prognosis. Although previous study involving angiosarcoma patients some of whom received radical surgery described that histologic grade is associated with prognosis [16], we did not find any association of histologic grade with prognosis in patients with advanced stages.
We observed that some of the patients responded to first-line wPac or later-line pazopanib who showed significantly prolonged survival. Although there had been no clinical trials comparing the outcome of palliative systemic chemotherapy versus best supportive care, these results indicate that there are subsets of patients who may benefit from the palliative first-line wPac and second-line pazopanib in terms of survival gain. The same has also been suggested in previous retrospective analysis [17]. We also demonstrated that patients without liver involvement might benefit from wPac compared with other regimens.
Our results on first-line wPac showed seemingly inferior efficacy compared with previous studies. A prospective phase II clinical trial on wPac produced ORR of 45.8%, PFS of 6.6 months, and OS of 19.5 months [18]. Retrospective studies on first-line wPac demonstrated ORR of 45.5%–52%, PFS of 5.6–5.7 months, and OS of 13.1–18.6 months [17,19]. However, these results could be partly due to relatively lower number of patients with poor ECOG PS and liver involvement enrolled in other studies. Indeed, we showed in our study that both of these clinical features were poor prognostic factors. Another previous phase II clinical trial on wPac for angiosarcoma patients showed ORR of 18%, PFS of 4 months and OS of 8 months, which were comparable with our results [7].
Efficacy of second- or later-line of pazopanib in our study showed inferior results compared with the previous study of phase II clinical trial of pazopanib for metastatic STS, which showed PFS of 4.6 months and OS of 12.5 months [12]. However, this result may be attributed to the higher portion of other STSs in the enrolled patients such as leiomyosarcoma and synovial sarcoma, which have generally better prognosis than angiosarcoma [1]. Anti-angiogenesis agents for angiosarcoma generally produced ORR up to 14% and PFS around 3.8–4.7 months, which are similar to our study results [1]. A retrospective study on pazopanib for advanced vascular sarcomas also exhibited comparable ORR of 20%, and PFS and OS of 3 and 9.9 months, respectively, in angiosarcoma [20]. A multicenter phase II prospective trial of pazopanib is ongoing to confirm these results (Clinical trial information: NCT01462630).
Angiosarcoma is an endothelial tumor in which all three subtypes of VEGF receptors can be overexpressed [9,10,21]. In addition, recurrent mutations in PTPRB and PLCG1 genes, which are intimately linked to angiogenesis, have also been identified in angiosarcoma [22]. Therefore, there has been great interest in targeting angiogenesis for angiosarcoma [1]. Both wPac and pazopanib have anti-angiogenesis effects. wPac exerts anti-angiogenesis effects by multiple mechanisms including inhibition of the release of VEGF and angiopoietin-1 by tumor cells [23], while pazopanib directly targets VEGFR1/2/3, platelet-derived growth factor receptor and several other key proteins responsible for angiogenesis [24]. Despite some efficacy of using anti-angiogenesis drugs in angiosarcoma patients, there seems to be more factors involved in resistance to such treatments in angiosarcoma [25].
In this study, we showed that liver involvement, either by primary mass or metastatic mass, was potentially associated with poor treatment outcome. Poor survival of hepatic angiosarcoma has been reported in association with various clinical features such as intraabdominal hemorrhage due to tumor rupture and acute liver failure [26,27]. We showed that poor survival associated with liver involvement may be partly due to poor liver function represented as pre-chemotherapy bilirubin and albumin level, which would have affected drug tolerability and patient’s performance status. However, tendency to poor PFS and ORR in patients with liver involvement implicate additional mechanisms on tumor biology. Such resistance mechanism of liver involvement by tumors has been also implicated in other cancers and other antitumor agents, such as lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors and melanoma to immune checkpoint inhibitors, which exhibited reduced CD8+ T cell density at the invasive tumor margin at pretreatment biopsies [28,29]. In addition, previous study showed that vessel co-option, a process whereby cancer incorporates pre-existing vessels from surrounding tissue, is a clinically relevant mechanism of resistance to anti-angiogenic therapy in liver metastases of colorectal and breast cancers [30]. Whether these mechanisms are also involved in angiosarcoma resistance would require further studies.
The limitations of this study include its retrospective nature; therefore, the results presented should be interpreted carefully. Also, as angiosarcoma can have diverse patterns of the clinical course according to involved sites, the ORR, the PFS, and the OS presented in this study would have been affected by the heterogeneity. However, due to the rarity of this disease, designing a clinical trial for advanced angiosarcoma is difficult, as observed in the previous phase II clinical trial, which enrolled 30 patients with heterogeneous primary sites [7]. In addition, most of the clinical trials involving angiosarcoma include other sarcomas, which results in even more heterogeneous study population with angiosarcoma being a small portion of the entire population. In this study, we collected data from homogeneous and relatively large number of patients in advanced setting who received palliative systemic chemotherapy. Therefore, our results provide clinicians with fair information of real-world treatment outcomes in advanced angiosarcoma patients. Secondly, whether liver function represented as more relevant scoring systems such as Child-Pugh or Model for End-Stage Liver Disease scores is correlated with clinical outcome should be further evaluated. Nevertheless, the baseline serum total bilirubin and albumin levels may provide clinical insights to physicians and researchers in deciding on treatments and designing stratifications for clinical trials. Lastly, our study implicates the liver involvement as a prognostic marker, not a predictive marker. Therefore, the presence or absence of liver involvement should not be used as a factor that could influence the decision on the initiation of chemotherapy.
In conclusion, treatment with first-line chemotherapy with wPac regimen and later-line pazopanib showed comparable efficacy to that reported in previous studies and may provide survival benefit for subset of patients, especially those without liver involvement. Clinicians would need to be aware that patients with presence of liver involvement may have poor prognosis. Further investigations with large number in multicenters or prospective design considering these results are warranted.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement All data collection and analyses were conducted after the review and approval of the institutional review board (IRB approval number: H-1807-198-967) and were done in compliance with the Declaration of Helsinki. Informed consent was not required for this study because of its retrospective nature. Author Contributions Conceived and designed the analysis: Park C, Kim M. Collected the data: Park C, Kim M, Kwak Y, Moon KC, Kim SH, Keam B, Kim YJ, Kim TM, Kim DW. Contributed data or analysis tools: Park C, Kim M, Kwak Y, Moon KC, Kim SH, Keam B, Kim YJ, Kim TM, Kim DW. Performed the analysis: Park C, Kim M. Wrote the paper: Park C, Kim M. AcknowledgmentsWe thank Juyoun Kim, a data manager at Seoul National University Hospital, who managed the database. We also thank Seonah Ha, PhD, who assisted in medical writing.
Fig. 1Overall survival of advanced angiosarcoma patients treated with systemic chemotherapy and the associated prognostic factors. Kaplan-Meier survival curves represent overall survival of advanced angiosarcoma patients. (A) Overall survival of patients included in the study. (B) Blue line represents patients without any liver involvement; red line represents patients with primary hepatic angiosarcoma; green line represents patients with liver metastasis. (C) Blue line represents patients without any liver involvement; red line represents patients with liver involvement. Censored data are marked with vertical segments and numbers at risk are demonstrated on the table at the bottom of each plot. 
Fig. 2Progression-free survival and overall survival of advanced angiosarcoma patients treated with first-line weekly paclitaxel (wT) or other regimens. Kaplan-Meier survival curves represent progression-free survival and overall survival of advanced angiosarcoma patients. (A, B) Survival curves show progression-free survival and overall survival of patients according to response to first-line wT. Blue line represents the progression-free survival of all patients who received first-line wT. Red dashed line represents non-responder and green dashed line represents responder. (C, D) Survival curves show progression-free survival and overall survival of patients without liver involvements according to the chemotherapy regimens. Blue line represents patients who received first-line wT and red line represents patients who received other regimens. Censored data are marked with cross segments and numbers at risk are demonstrated on the table at the bottom of each plot. 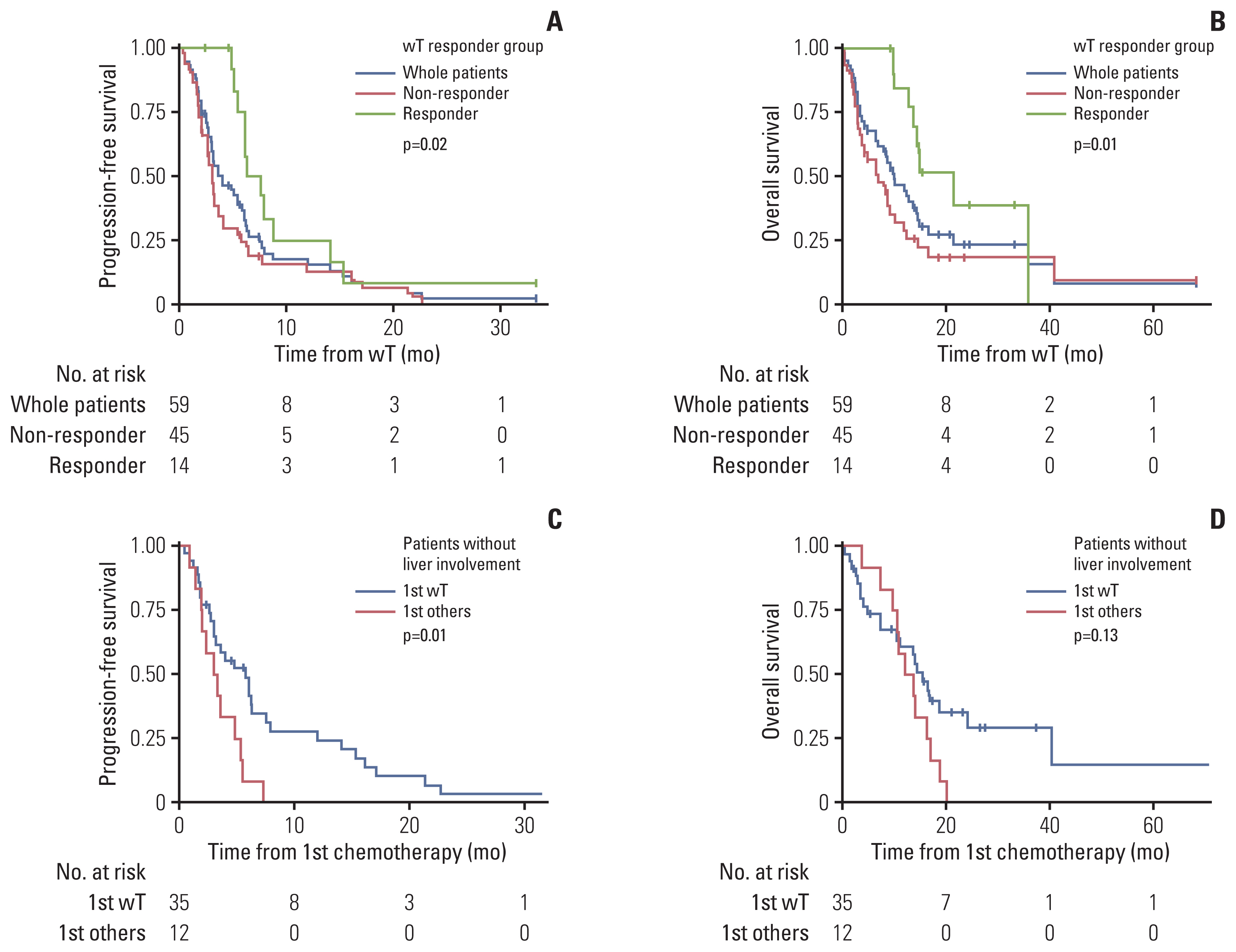
Fig. 3Progression-free survival and overall survival of advanced angiosarcoma patients treated with second- or later-line pazopanib. Kaplan-Meier survival curves represent progression-free survival and overall survival of advanced angiosarcoma patients. (A, B) Survival curves show progression-free survival and overall survival of patients according to response to second- or later-line pazopanib. Blue lines represent the progression-free survival and overall survival of all patients who received first-line weekly paclitaxel. Red lines represent non-responder and green lines represent responder. Censored data are marked with cross segments and numbers at risk are demonstrated on the table at the bottom of each plot. 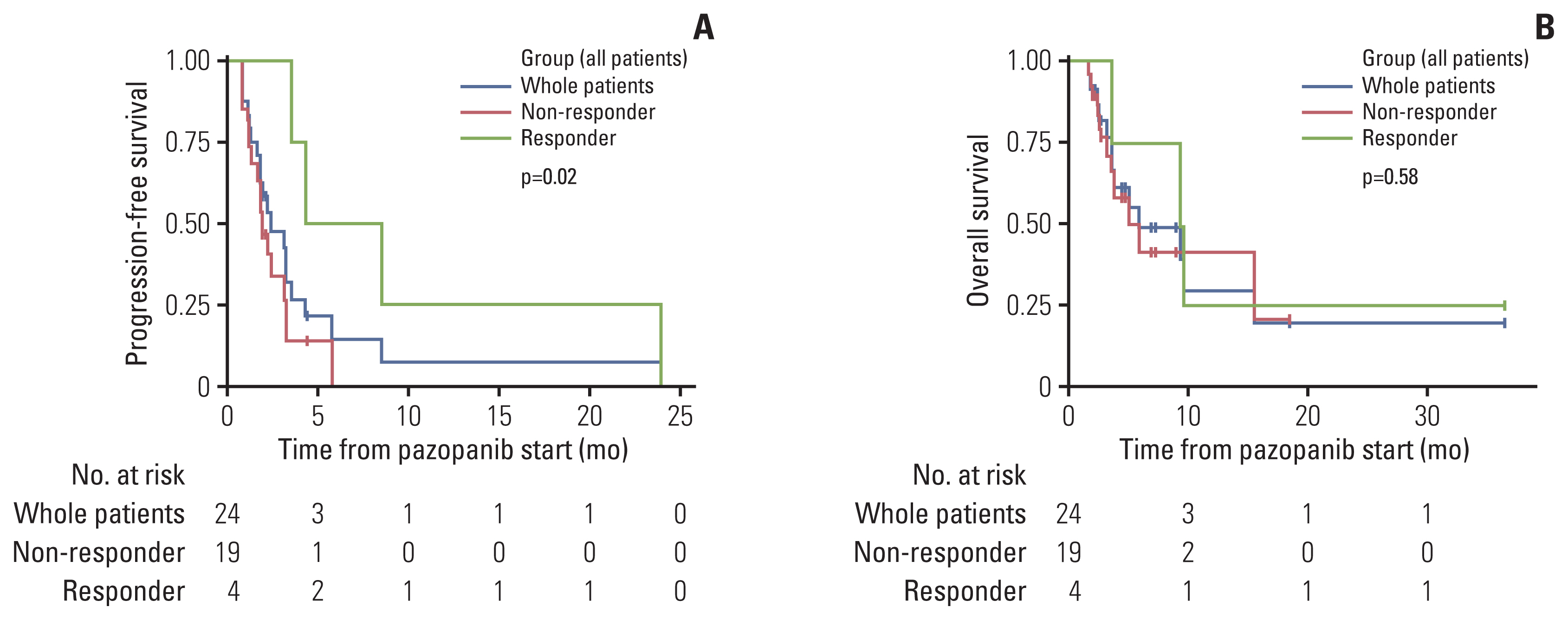
Table 1Demographic features of patients
Table 2Cox proportional hazard analysis of the prognostic factors of overall survival in patients with advanced angiosarcoma at the time of the first chemotherapy References2. Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251:1098–106.
3. Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, Raskin KA, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953–67.
4. Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030–6.
5. Penel N, Marreaud S, Robin YM, Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol. 2011;80:257–63.
6. Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178–86.
7. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–74.
8. Weidema ME, Versleijen-Jonkers YM, Flucke UE, Desar IM, van der Graaf WT. Targeting angiosarcomas of the soft tissues: a challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120–31.
9. Tokuyama W, Mikami T, Masuzawa M, Okayasu I. Autocrine and paracrine roles of VEGF/VEGFR-2 and VEGF-C/VEGFR-3 signaling in angiosarcomas of the scalp and face. Hum Pathol. 2010;41:407–14.
10. Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol. 2008;97:74–81.
11. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257–63.
12. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86.
13. Fletcher CD, Brdige JA, Hogendoorn PC, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
15. Common terminology criteria for adverse events, v5.0 [Internet]. Waltham, MA: UpToDate; 2017. [cited 2018 May 1]. Available from: http://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events
16. Wang L, Lao IW, Yu L, Wang J. Clinicopathological features and prognostic factors in angiosarcoma: A retrospective analysis of 200 patients from a single Chinese medical institute. Oncol Lett. 2017;14:5370–8.
17. Penel N, Italiano A, Ray-Coquard I, Chaigneau L, Delcambre C, Robin YM, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517–23.
18. Ray-Coquard IL, Domont J, Tresch-Bruneel E, Bompas E, Cassier PA, Mir O, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797–802.
19. Byeon S, Song HN, Kim HK, Ham JS, Lee SJ, Lee J, et al. A Korean single-center, real-world, retrospective study of first-line weekly paclitaxel in patients with metastatic angiosarcoma. Clin Sarcoma Res. 2016;6:8.
20. Kollar A, Jones RL, Stacchiotti S, Gelderblom H, Guida M, Grignani G, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88–92.
21. Zietz C, Rossle M, Haas C, Sendelhofert A, Hirschmann A, Sturzl M, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. 1998;153:1425–33.
22. Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet. 2014;46:376–9.
23. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16:481–92.
24. Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2011;77:163–71.
25. Wagner MJ, Ravi V, Menter DG, Sood AK. Endothelial cell malignancies: new insights from the laboratory and clinic. NPJ Precis Oncol. 2017;1:11.
26. Lee SW, Song CY, Gi YH, Kang SB, Kim YS, Nam SW, et al. Hepatic angiosarcoma manifested as recurrent hemoperitoneum. World J Gastroenterol. 2008;14:2935–8.
27. Bhati CS, Bhatt AN, Starkey G, Hubscher SG, Bramhall SR. Acute liver failure due to primary angiosarcoma: a case report and review of literature. World J Surg Oncol. 2008;6:104.
28. Chang YP, Chen YM, Lai CH, Lin CY, Fang WF, Huang CH, et al. The impact of de novo liver metastasis on clinical outcome in patients with advanced non-small-cell lung cancer. PLoS One. 2017;12:e0178676.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||