AbstractPurposeThe epidemiology of B-cell non-Hodgkin lymphoma (BNHL) in Asia is not well described, and rates of second primary malignancies (SPM) in these patients are not known. We aimed to describe temporal changes in BNHL epidemiology and SPM incidence in Korea.
Materials and MethodsA retrospective cohort study used claims data from the National Health Insurance Service that provides universal healthcare coverage in Korea. Newly diagnosed patients aged at least 19 years with a confirmed diagnosis of one of six BNHL subtypes (diffuse large cell B-cell lymphoma [DLBCL], small lymphocytic and chronic lymphocytic [CLL/SLL], follicular lymphoma [FL], mantle cell lymphoma [MCL], marginal zone lymphoma [MZL], and lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia [WM]) during the period 2006-2015 were enrolled and followed up until death, dis-enrolment, or study end, whichever occurred first. Patients with pre-existing primary cancers prior to the diagnosis of BNHL were excluded.
ResultsA total of 19,500 patients with newly diagnosed BNHL were identified out of 27,866 with non-Hodgkin lymphoma (NHL). DLBCL was the most frequently diagnosed subtype (41.9%-48.4% of NHL patients annually, 2011-2015). Standardized incidence of the six subtypes studied per 100,000 population increased from 5.74 in 2011 to 6.96 in 2015, with most increases in DLBCL, FL, and MZL. The incidence (95% confidence interval) of SPM per 100 person-years was 2.74 (2.26-3.29) for CLL/SLL, 2.43 (1.57-3.58) for MCL, 2.41 (2.10-2.76) for MZL, 2.23 (2.07-2.40) for DLBCL, 1.97 (1.61-2.38) for FL, and 1.41 (0.69-2.59) for WM.
IntroductionB-cell non-Hodgkin lymphoma (BNHL) comprises a heterogeneous group of lymphoid malignancies that differ in their clinical presentation and progression [1]. The most common type of mature BNHL in adults is diffuse large B-cell lymphoma (DLBCL), which comprises approximately 46% of mature BNHL in Korea [2]. Other subtypes include follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL, including mucosa-associated lymphoid tissue [MALT] lymphoma), chronic lymphocytic and small lymphocytic lymphoma (CLL/SLL), and Waldenström’s macroglobulinemia (WM or lymphoplasmacytic lymphoma). BNHL subtypes vary in their ethnic and regional distribution, possibly influenced by genetic, lifestyle, and environmental factors [1,3].
The prognosis of BNHL has improved in the last decade, attributed primarily to the addition of rituximab to standard chemotherapy regimens [4]. Depending on subtype, some patients will be cured with treatment and others will survive for longer than a decade [1]. Prolonged survival is associated with its own complications, such as the development of second primary malignancies (SPM). A meta-analysis of 23 studies reported that SPM occur in patients with non-Hodg-kin’s lymphoma significantly more frequently than primary malignancies in the general population, with a relative risk estimated at 1.88 (95% confidence interval [CI], 1.58 to 2.22) [5]. However, most of the data came from North American and European populations, and only two Japanese studies were included from the Asian region. Exposure to chemotherapy, autologous stem cell transplant, total body irradiation, and younger age at diagnosis have all been implicated as risk factors in the development of SPM [5,6].
The incidence of BNHL is reportedly lower in Asian than in Western countries and the distribution of BNHL subtypes is also different [1,2]. Compared to Western countries, a higher proportion of patients in Asia with BNHL have MZL, and lower proportions have FL and CLL/SLL [7-9]. Some of these differences likely reflect known variations in genetic susceptibility to BNHL between Asian and Western populations [10,11]. However, for some subtypes, other mechanisms, such as different molecular pathways or etiologic factors are thought to contribute to regional differences in incidence rates. For example, while the incidence of FL is lower in Asian than in Western populations, the occurrence of the characteristic bcl-2 translocation found in FL is similar in healthy populations in both regions, suggesting that the development of FL may be triggered differently in Asia than in Western countries [12].
The Republic of Korea is a high-income, industrialized country that lies to the East of the Asian mainland, with a population of approximately 52 million. The Korean government began national health insurance programs in 1976, expanded their coverage to all citizens in 1989, and created a single-payer system (Korean National Health Insurance System, NHIS) from 2000 [13]. Those insured pay contributions and receive medical services from their healthcare providers. The National Health Information Database (NHID) is maintained by the NHIS and contains all medical and prescription drug claim records for the Korean population, and is a rich repository of information for health research [14].
Between 1999 and 2012, the age-standardized incidence of mature BNHL increased annually by 5.6% [2]. The rate of SPM among patients with BNHL in Korea is not known. To address this knowledge gap, we conducted a retrospective cohort study using claims data from the NHID to describe temporal changes in the incidence and prevalence of BNHL in Korea up until 2015, and to evaluate the incidence of SPM in patients with BNHL.
Materials and Methods1. Data sourceThe NHID holds comprehensive information on health care utilization, demographic characteristics, and mortality for the whole population of South Korea [13,14]. The NHID was built in 2012 to perform NHIS activities using information from medical treatment and health screening records, and socio-demographic data from an existing database system [14]. Inpatient and outpatient visits including diagnoses recorded in International Classification of Diseases, 10th revision (ICD-10) format, length of stay, treatment costs, procedures, and prescriptions are recorded [13,14].
2. Study populationAll patients who were newly diagnosed with one of six BNHL subtypes (ICD-10 codes C83.3 DLBCL, C91.1 CLL/SLL, C82 FL, C83.1 MCL, C88.4 MALT lymphoma and C83.0 MZL, or C88.0 WM) between 01 January 2006 and 31 December 2015 were identified in the NHID. Nodal and extra-nodal MZL were included under one category because they are unable to be differentiated under C83.0. Patients were defined as newly diagnosed if they had no previous record of diagnosis or treatment of BNHL for at least 1 year. The diagnosis of BNHL was confirmed if the patient had at least three outpatient visits, and/or one hospital admission with the specific ICD-10 code after the diagnosis index date.
Patients were included in the study cohort if they were at least 19 years of age and if they had been in the database for at least 12 months prior to the BNHL diagnosis index date. Patients were excluded if they had any cancer other than BNHL prior to the diagnosis index date.
SPM were defined as new malignant tumors diagnosed more than 180 days after the BNHL index date to reduce the risk of including pre-existing undiagnosed malignancies in the analysis [15], SPM were identified in the NHID. Patients were followed up for SPM from the diagnosis index date until death, end of the study period, or dis-enrolment, whichever occurred first. All kinds of solid tumors were considered as SPM. For hematologic malignancies as SPM, we included acute myeloid leukemia (C92), monocytic leukemia (C93), myelodysplastic syndrome (D46), and myeloproliferative neoplasms (D47), but excluded acute leukemia of unspecified cell type (C95), multiple myeloma (C90) and all subtypes of lymphoma.
Since there have been changes in the diagnostic codes for BNHL subtypes in the NHIS over time, the study period was divided into two parts: years 2006 to 2010 and years 2011 to 2015. The cohort analysis of incidence and prevalence was conducted using data from 2011-2015, whereas the full dataset from 2006 to 2015 was used to estimate the incidence of SPM.
3. Statistical analysisThe incidence of BNHL for each study year was calculated as the number of newly diagnosed patients with BNHL divided by the total Korean population in each calendar year. Prevalence included all patients with existing and incident BNHL divided by the total Korean population in each calendar year. Mortality rates for BNHL and its six subtypes were calculated as the number of patient deaths due to any cause divided by the total Korean population in each calendar year. Age-standardized rates were calculated by the direct standardization method, using the age distribution of the Korean population in 2011 as the standard population. Overall survival (OS) was calculated using Kaplan-Meier methods.
The number of patients with at least one SPM was determined and the incidence calculated using the number of patients divided by the sum of the total follow-up period (per 100 person-years). Kaplan-Meier survival curves for SPM used the first SPM event for the time-to-event analysis. Cochran-Armitage tests were used to describe trends in the data over time. All analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
4. Ethical statementAll personally identifiable information was encrypted to protect patient privacy. The study protocol was reviewed and received approval from the Institutional Review Board of Yonsei University Health System, Severance Hospital with a waiver of informed consent (4-2016-0824), and the National Health Information Data Request Review Committee of the NHIS (REQ0000006774).
ResultsA total of 19,500 patients with BNHL in the NHID had a diagnosis index date between 2006 and 2015 out of 27,866 with NHL (Table 1). Of these, 13,671 were diagnosed between 2011-2015 and were included in the cohort analysis. The mean age of patients diagnosed between 2011 and 2015 was 59.5 years, 60.0% (n=8,202) of patients were 19-64 years of age and 54.0% (n=7,389) were male.
The total number of BNHL cases diagnosed increased annually from 2,298 in year 2011 up to 3,128 in year 2015. DLBCL was the most common diagnosis in each study year (Table 1), although the relative proportion of DLBCL tended to decrease over time from 43.0% of all NHL diagnoses in 2011 to 43.4% in 2014 (but increasing to 48.4% in 2015). The proportion of CLL/SLL decreased during the study, from 4.6% of NHL in 2011 to 3.7% in 2015. By contrast, the proportion of FL and MZL increased between 2011 and 2015 (6.4% to 7.8% for FL and MZL 16.6% to 21.7% for MZL). There was little change in the proportion of patients diagnosed with NHL with MCL or WM over time.
1. Incidence, prevalence, and all-cause mortality rates in patients with BNHLThe age-standardized incidence of BNHL increased over time from 5.74 (95% CI, 5.51 to 5.98) per 100,000 population in 2011 to 6.96 (95% CI, 6.72 to 7.20) per 100,000 population in 2015 (Table 2). Age-standardized incidence rates of DLBCL, MZL, and FL increased significantly (p < 0.001 for all), with similar increases observed in men and women (Fig. 1). Between 2011 and 2015, the incidence of DLBCL increased by 11%, the incidence of MZL increased by 32%, and the incidence of FL increased by 25%. Age-standardized incidence rates of MCL and WM remained steady between 2011 and 2015, although there was some annual variation in the incidence in women versus men for WM. A decrease in the incidence of CLL/SLL appeared in 2015 (Fig. 1).
Crude and age-standardized BNHL prevalence increased steadily each year and were approximately 2.5-fold higher in 2015 than in 2011 (Table 2). Prevalence rates of each BNHL subtype also increased (Fig. 2). The age-adjusted prevalence of DLBCL increased by 1.8-fold, CLL/SLL by 1.7-fold, FL by 2.6-fold, MCL by 4.0-fold, MZL by 11.3-fold and WM by 1.6-fold (p < 0.001 for all six subtypes). Increases appeared to be similar in women and men for all BNHL subtypes.
Crude and age-standardized BNHL mortality rates increased over time. Age-standardized mortality increased by 42%, from 1.33 (95% CI, 1.22 to 1.44) per 100,000 population in 2011 to 1.89 (95% CI, 1.76 to 2.02) per 100,000 population in 2015 (Table 2). Age-standardized mortality increased among men and women for all BNHL subtypes; except for women with WM in whom the mortality rate remained rather constant (Fig. 3). The increase in age-standardized mortality was statistically significant for DLBCL (p=0.010), and CLL/SLL, MCL, MZL (p < 0.001). Between 2011 and 2015, all-cause mortality rates increased by 35% in patients with for DLBCL, 49% in patients with CLL/SLL, 31% in patients with FL, 172% in MCL, 77% in MZL and 121% in WM.
2. Overall survivalFive-year OS was highest in patents with MZL (88%) followed by FL (79%), CLL/SLL (62%), DLBCL (60%), WM (54%), and MCL (53%) (Table 3, Fig. 4). Up to three years after diagnosis, DLBCL patients had the lowest survival rate. However, when followed for a longer duration, MCL and WM subtypes showed lower survival rates than DLBCL.
3. Occurrence of SPMOf the 19,500 patients diagnosed with BNHL in 2006-2015, 1,183 patients (6.1%) developed a total of 1,203 SPMs. The incidence of the first SPM was highest in patients with CLL/SLL (2.74 per 100 person-years), followed by MCL (2.43 per 100 person-years), MZL (2.41 per 100 person-years), DLBCL (2.23 per 100 person-years), FL (1.97 per 100 person-years), and WM (1.41 per 100 person-years) (Table 4).
The incidence of solid SPM was highest in patients with MCL (2.32 per 100 person-years) (Table 4). The most frequent solid malignancies were prostate cancer in 190 patients (0.64 per 100 person-years; 95% CI, 0.55 to 0.73), liver cancer in 234 patients (incidence rate 0.43 per 100 person-years; 95% CI, 0.38 to 0.49), stomach cancer in 174 patients (0.32 per 100 person-years; 95% CI, 0.28 to 0.37), colorectal cancer in 167 patients (0.31 per 100 person-years; 95% CI, 0.26 to 0.36), lung cancer in 139 patients (0.26 per 100 person-years; 95% CI, 0.22 to 0.30), brain cancer in 88 patients (0.16 per 100 person-years; 95% CI, 0.13 to 0.20), pancreatic cancer in 84 patients (0.15 per 100 person-years; 95% CI, 0.12 to 0.19), ovarian cancer in 37 patients (0.15 per 100 person-years; 95% CI, 0.11 to 0.21), breast cancer in 33 patients (0.13 per 100 person-years; 95% CI, 0.09 to 0.19), and head and neck cancer in 59 patients (0.11 per 100 person-years; 95% CI, 0.08 to 0.14).The cumulative incidence at 5 years for developing the first SPM was 15% for CLL/SLL, 12% for MCL and MZL, 11% for DLBCL, 9% for FL, and 7% for WM (Table 5).
A sensitivity analysis conducted in which the SPM analysis was restricted to 2011-2015 after implementation of changes in the diagnostic codes for BNHL subtypes showed similar results compared to the entire study period from 2006-2015 (data not shown).
DiscussionTo our knowledge, this is the first analysis of the incidence of SPM in Korean patients with BNHL. By analyzing virtually all cases of BNHL in the Korean health system, we found a high rate of progression to SPM, with a 5-year cumulative incidence of 7% to 15% depending on subtype after the BNHL diagnosis, and evidence of progression to SPM that continued past the 9-year follow-up period of our study. This contrasts with 5-year cumulative incidence of approximately 3% for patients with early stage Hodgkin’s lymphoma [16], and 6% for patients with multiple myeloma [17].
Our estimates of the cumulative incidence of SPM are somewhat higher than reported elsewhere. Studies in the United States using the Surveillance, Epidemiology, and End Results (SEER) database report 5- and 10-year cumulative incidence of SPM of 5.68% and 11.06%, respectively for patients with FL, and 9.5% and 16.1% for patients with WM [18,19]. Another SEER study reported that 8.2% of patients with MCL developed a SPM, although the median follow-up was only 31 months [20]. The 5- and 10-year cumulative incidences of SPM in patients with DLBCL was 5.41% and 10.47% in California in the post-rituximab era, which was higher than in the years prior to rituximab introduction [21]. At this time, we have no clear view of why SPM may be more frequent in Korean patients with BNHL than elsewhere, although different follow-up periods, study populations, methodologies and possibly treatment regimens are likely to have contributed. Similar data on SPM rates in patients with BNHL from Asian countries are limited, and we cannot confirm whether the rates of SPM observed in our study are reflective of trends present across Asia. Corroborative data from other parts of Asia as well as studies investigating the genetic predispositions associated with BNHL in Asia populations could help inform these findings. In 2015 the age-standardized cancer incidence rate in Korea was 258.9 per 100,000 population, and the lifetime cumulative risk of developing cancer was 35.3% [22]. However, the cumulative lifetime risk of cancer is expected to increase as because the average lifespan of Koreans continues to increase [23].
Consistent with previous reports, the age-standardized incidence rate of BNHL in Korea was substantially lower than those observed in Western countries, although direct comparisons should be made cautiously given that the data were not standardized using the same reference population [2,24]. The incidence of DLBCL was an exception. In Europe, the age-standardized incidence of DLBCL in 2000-2002 was 3.13 per 100,000, which is lower than our 2015 estimate of 3.67 per 100,000 population, the highest incidence yet recorded in Korea [24]. The European age-standardized incidence was 3.79 per 100,000 for CLL/SLL, and 1.92 per 100,000 for FL, compared with 2015 estimates of 0.27 and 0.63 per 100,000 respectively in Korea. In Canada, the incidence of mature BNHL was 54.0 per 100,000 population in men, and 38.5 per 100,000 in women between 2010-2013 [25], compared to an overall rate of < 7 per 100,000 population during the same years in Korea.
The age-adjusted incidence of BNHL increased by 17% from 2011 to 2015. The increase in incidence was driven by changes in DLBCL, MZL, and FL, which increased by 17%, 40%, and 36%, respectively, over the study period. In parallel, there was a 16% decrease in the incidence of CLL/SLL. The changes in disease incidence appeared to be similar in men and women. Our observations compliment and extend those reported by Lee et al. [2] using data from the Korean Central Cancer Registry and confirm the steady increase in the age-standardized incidence of DLBCL, MZL, and FL in Korea over the last 16 years (Fig. 5). While we cannot exclude that some of the observed increases may be due to improved surveillance or diagnostic methods, marked increases in the incidence of DLBCL and FL have been observed previously in other countries including Canada and the United States [25,26]. Age-standardized mortality rates in patients with BNHL increased in the Korean population over the study period reflecting increases in incidence and prevalence.
The distribution of BNHL subtypes in our study was somewhat different to that reported previously by Yoon et al. [8] from a single large institution in Korea using data from 1989 to 2008. Compared to Yoon et al. [8], we observed higher proportions of patients with CLL/SLL (4.4% to 6.2% vs. 3.7%), FL (8.1% to 9.8% vs. 3.4%) and WM (0.9% to 1.3% vs. 0.5%). Estimates for the other subtypes were within the same range. Differences for some subtypes could reflect the case definition used by Yoon et al. [8] which was laboratory-based with exclusion of patients without pathological material available for review, in contrast to our study that used ICD-10 codes arising from claims data. Additionally, our study covered a later period, from 2006 to 2015, allowing us to include more patients from recent years.
Lee et al. [2], explored the epidemiology of lymphoid malignancies in Korea using data from the Korean Central Cancer Registry between 1999 and 2012. The age-standardized incidence rate of mature BNHL increased significantly (5.6% annually) during the study and was 6.60 per 100,000 in 2012. Significant increases were also observed for MCL (7.1% annual increase), FL (5.1% annual increase), DLBCL (4.0% annual increase), and MZL (18.4% annual increase).
Strengths of our study include the use of a national health insurance database which ensured we captured essentially all cases of BNHL and SPMs diagnosed in Korea during the study years. The 10-year study duration was important for detecting SPM in view of the long latency between BNHL diagnosis and the development of SPM. Median latency periods from diagnosis until the development of SPM are approximately 4 years [18,20].
Potential study limitations included the lower accuracy of coding information in the years 2006-2010, which meant that we restricted the cohort analysis of incidence and prevalence to 5 years from 2011. In addition, patients who achieved a cure after appropriate treatment might be included in the calculation of prevalence in this study because we were not able to identify the exact disease status. Therefore, prevalence might be overestimated. The Korean health insurance database does not differentiate between malignancies that are primary or metastatic. By excluding patients with a previous cancer diagnosis, solid tumors diagnosed more than 180 days after the BNHL index date were likely to be SPM. For hematological malignancies it was not possible to distinguish between disease progression/transformation and a new primary hematological cancer. We excluded unspecified acute leukemia, multiple myeloma, and all subtypes of lymphoma to reduce the risk of over-estimating hematological SPM. However, potential overestimation of the incidence of SPM cannot be ruled out. Nevertheless, in view of the different epidemiology of BNHL in Asia compared to Western countries, our findings potentially reflect an important trend of increased SPM in Asia that warrants further investigation.
In conclusion, the incidence of BNHL in Korea continues to increase, driven by increases in MZL, FL, as well as DLBCL. Our findings present new challenges for clinicians managing BNHL. High incidences of SPM in patients with BNHL warrant careful ongoing review of these patients to ensure early diagnosis and treatment. BNHL and the long-term complications associated with survival remain important unmet medical needs in South Korea.
Conflicts of InterestYL, HQ, and LAR are employees of Janssen Research & Development LLC. YL, HQ, and LAR report stock ownership in Johnson & Johnson Pte Ltd. HCK, KHH, and JSK declare no conflicts of interest. Writing assistance was provided by Joanne Wolter (independent on behalf of Johnson & Johnson Pte Ltd). The work was supported by Janssen Research & Development LLC (Titusville, New Jersey, United States). Fig. 1.Age-standardized incidence rates (95% confidence intervals) of B-cell non-Hodgkin lymphoma subtypes in South Korea, 2011-2015. Age-standardized rates were calculated by direct standardization method, using the 2011 Korean population as the reference. p-values for trend: DLBCL p < 0.001 (A), MZL p < 0.001 (B), CLL/SLL p=0.489 (C), MCL p=0.077 (D), FL p < 0.001 (E), WM p=0.661 (F). DLBCL, diffuse large B-cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; MCL, mantle cell lymphoma; FL, follicular lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. 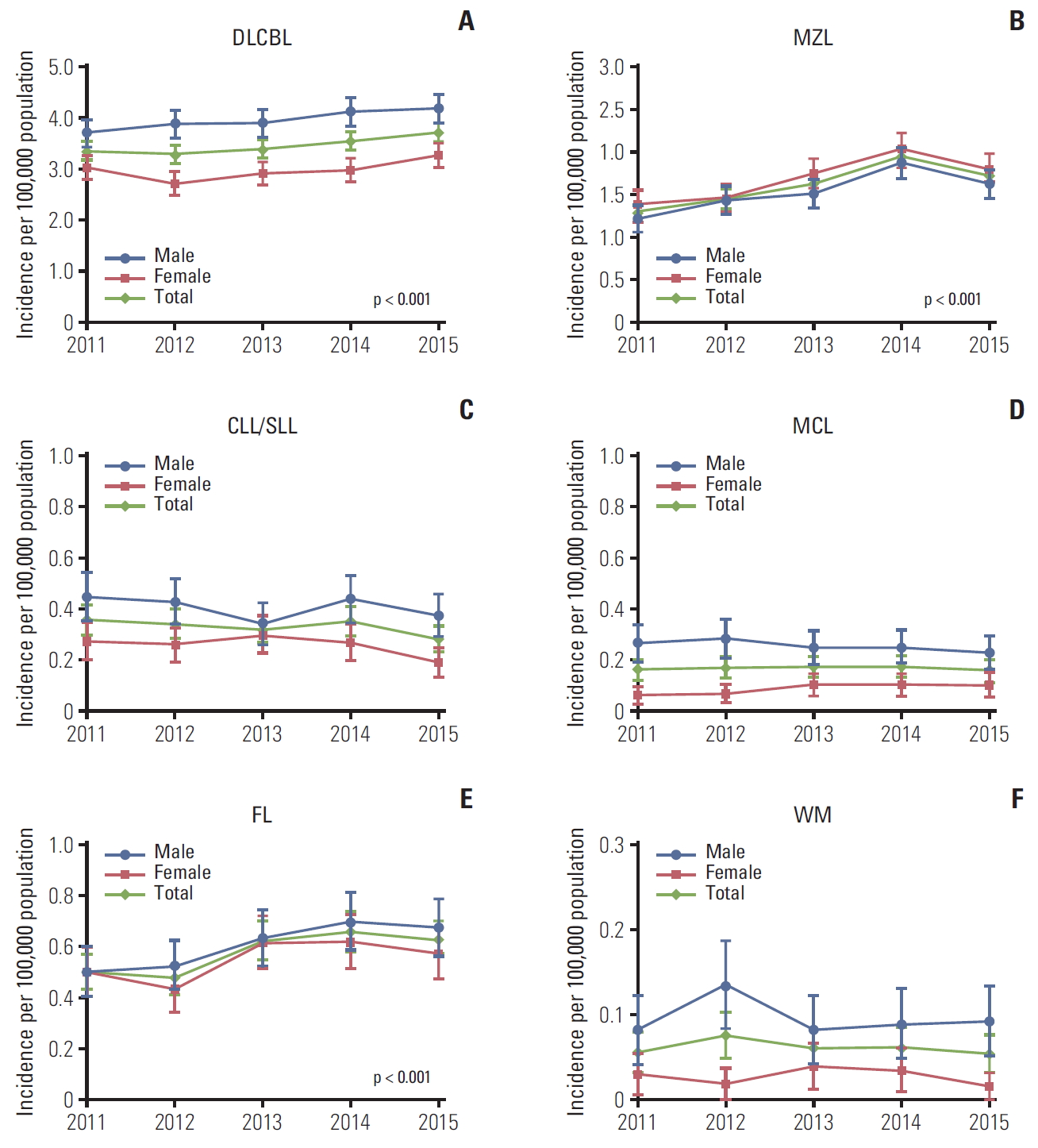
Fig. 2.Age-standardized prevalence (95% confidence intervals) of B-cell non-Hodgkin lymphoma subtypes in South Korea, 2011-2015. Age-standardized rates were calculated by direct standardization method, using the 2011 Korean population as the reference. p-values for trend: DLBCL p & 0.001 (A), MZL p & 0.001 (B), CLL/SLL p & 0.001 (C), MCL p & 0.001 (D), FL p & 0.001 (E), WM p & 0.001 (F). DLBCL, diffuse large B-cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; MCL, mantle cell lymphoma; FL, follicular lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. 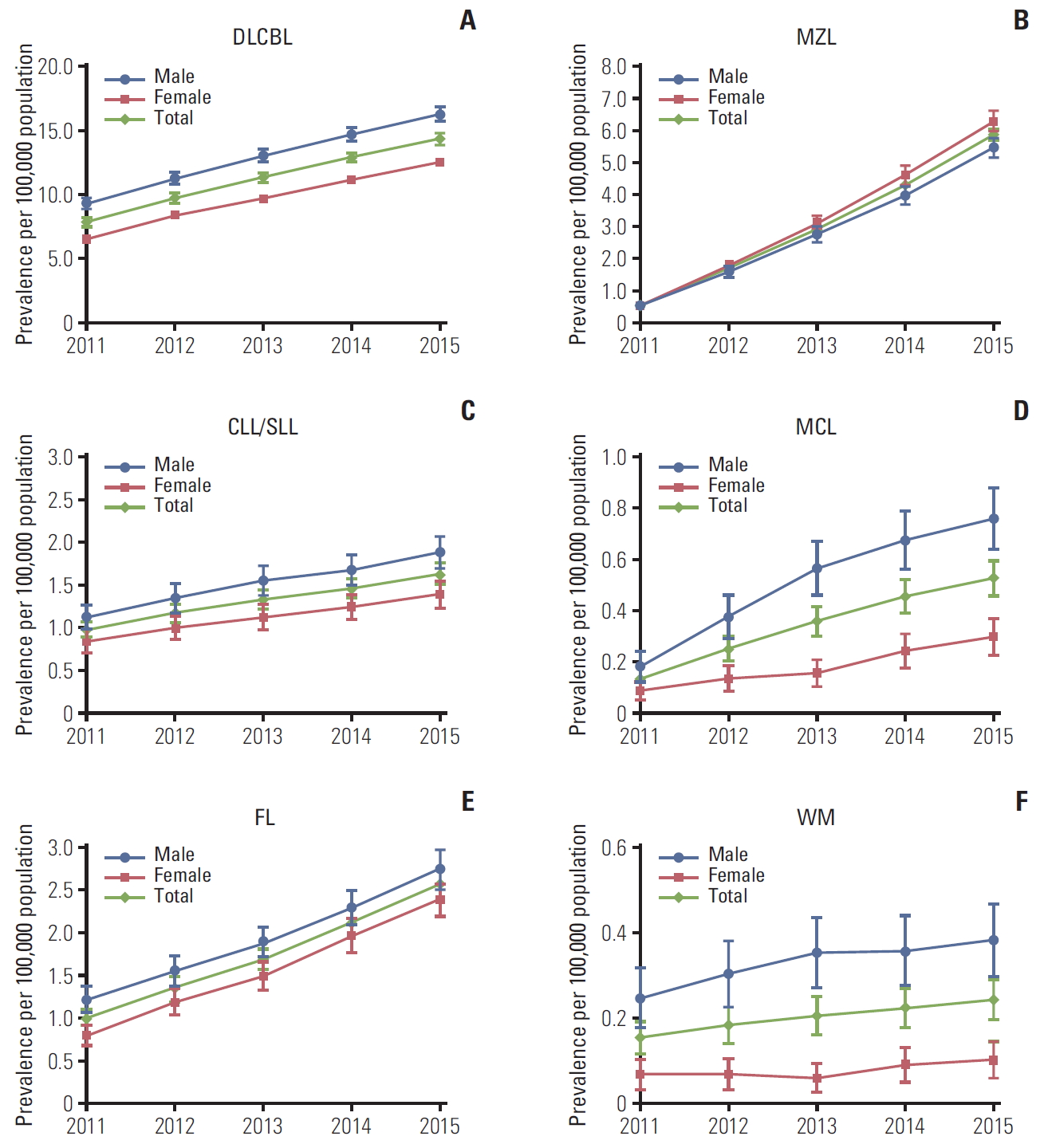
Fig. 3.Age-standardized all-cause mortality rates (95% confidence intervals) in patients with B-cell non-Hodgkin lymphoma subtypes in the Korean population, 2011-2015. Age-standardized rates were calculated by direct standardization method, using the 2011 Korean population as the reference. p-values for trend: DLBCL p < 0.010 (A), MZL p < 0.001 (B), CLL/SLL p < 0.001 (C), MCL p < 0.001 (D), FL p=0.937 (E), WM p=0.923 (F). DLBCL, diffuse large B-cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; MCL, mantle cell lymphoma; FL, follicular lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. 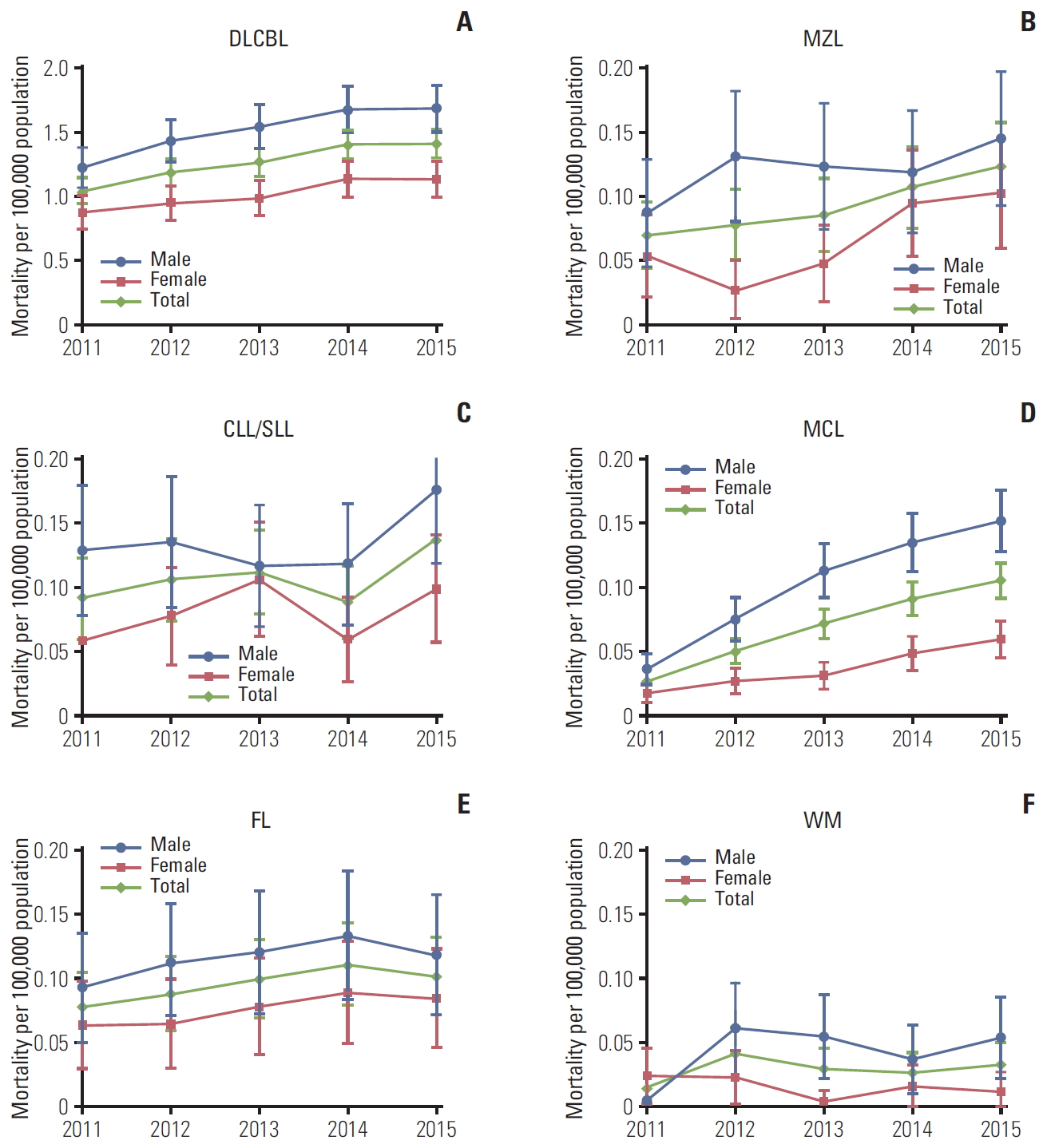
Fig. 4.Overall survival after B-cell non-Hodgkin lymphoma diagnosis index date: follow-up of all patients enrolled from 2006-2015 and death from any cause. MZL, marginal zone lymphoma; FL, follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; MCL, mantle cell lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. 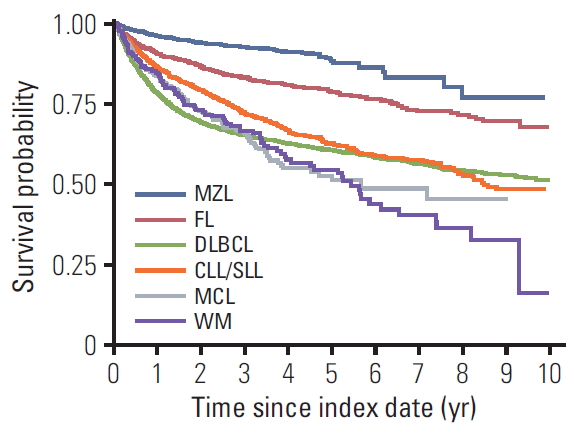
Fig. 5.Age-standardized incidence of B-cell non-Hodgkin lymphoma subtypes as reported fan Central Cancer Registry from 1999-2012 [2] and the National Health Information database from 2011-2015 (shaded). DLBCL, diffuse large B-cell lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma. 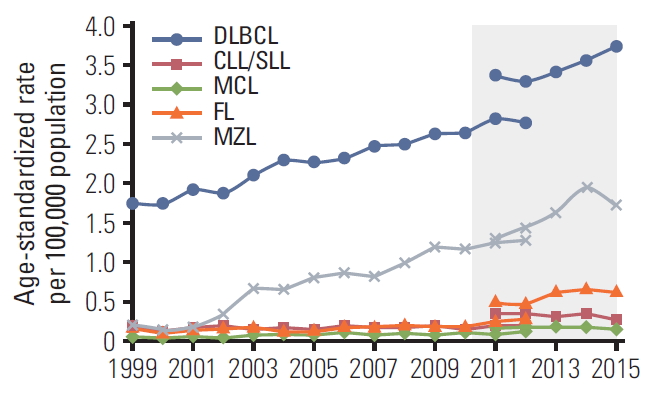
Table 1.New diagnoses of non-Hodgkin B-cell lymphomas in patient over the 2006 to 2015 study period Values are presented as number (%). DLBCL, diffuse large B-cell lymphoma; CLL/SLL, small lymphocytic lymphoma and chronic lymphocytic; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma; BNHL, B-cell non-Hodgkin lymphoma; NHL, non-Hodgkin lymphoma. %=percentage of all NHL Table 2.Crude and age-standardized incidence, prevalence and all-cause mortality rates of BNHL in the Korean population
Table 3.Overall survival in patients with different BNHL subtypes (2006-2015) BNHL, B-cell non-Hodgkin lymphoma; CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; CLL/SLL, chronic lymphocytic and small lymphocytic lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. Table 4.Incidence rate of second primary malignancies by BNHL subtype BNHL, B-cell non-Hodgkin lymphoma; CI, confidence interval; SPM, second primary malignancy; DLBCL, diffuse large B-cell lymphoma; CLL/SLL, chronic lymphocytic and small lymphocytic lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. a)Acute myeloid leukemia (C92), monocytic leukemia (C93), myelodysplastic syndrome (D46), or myeloproliferative neoplasms (D47). Table 5.Cumulative incidence (95% CI) for developing a SPM among patients with BNHL subtypes CI, confidence interval; SPM, second primary malignancy; BNHL, B-cell non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; CLL/SLL, chronic lymphocytic and small lymphocytic lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström’s macroglobulinemia or lymphoplasmacytic lymphoma. References2. Lee H, Park HJ, Park EH, Ju HY, Oh CM, Kong HJ, et al. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res Treat. 2018;50:222–38.
3. Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014:130–44.
4. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11:1327–42.
5. Pirani M, Marcheselli R, Marcheselli L, Bari A, Federico M, Sacchi S. Risk for second malignancies in non-Hodgkin’s lymphoma survivors: a meta-analysis. Ann Oncol. 2011;22:1845–58.
6. Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–15.
7. Perry AM, Diebold J, Nathwani BN, MacLennan KA, Muller-Hermelink HK, Bast M, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244–50.
8. Yoon SO, Suh C, Lee DH, Chi HS, Park CJ, Jang SS, et al. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol. 2010;85:760–4.
9. Chuang SS, Chen SW, Chang ST, Kuo YT. Lymphoma in Taiwan: review of 1347 neoplasms from a single institution according to the 2016 Revision of the World Health Organization Classification. J Formos Med Assoc. 2017;116:620–5.
10. Bassig BA, Cerhan JR, Au WY, Kim HN, Sangrajrang S, Hu W, et al. Genetic susceptibility to diffuse large B-cell lymphoma in a pooled study of three Eastern Asian populations. Eur J Haematol. 2015;95:442–8.
11. Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood. 2015;126:2265–73.
12. Biagi JJ, Seymour JF. Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood. 2002;99:4265–75.
13. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–28.
14. Seong SC, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800.
15. Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961;14:221–30.
16. LeMieux MH, Solanki AA, Mahmood U, Chmura SJ, Koshy M. Risk of second malignancies in patients with early-stage classical Hodgkin’s lymphoma treated in a modern era. Cancer Med. 2015;4:513–8.
17. Engelhardt M, Ihorst G, Landgren O, Pantic M, Reinhardt H, Waldschmidt J, et al. Large registry analysis to accurately define second malignancy rates and risks in a well-characterized cohort of 744 consecutive multiple myeloma patients followed-up for 25 years. Haematologica. 2015;100:1340–9.
18. Castillo JJ, Gertz MA. Secondary malignancies in patients with multiple myeloma, Waldenstrom macroglobulinemia and monoclonal gammopathy of undetermined significance. Leuk Lymphoma. 2017;58:773–80.
19. Giri S, Bhatt VR, Verma V, Pathak R, Bociek RG, Vose JM, et al. Risk of second primary malignancies in patients with follicular lymphoma: a United States population-based study. Clin Lymphoma Myeloma Leuk. 2017;17:569–74.
20. Shah BK, Khanal A. Second primary malignancies in mantle cell lymphoma: a US population-based study. Anticancer Res. 2015;35:3437–40.
21. Tao L, Clarke CA, Rosenberg AS, Advani RH, Jonas BA, Flowers CR, et al. Subsequent primary malignancies after diffuse large B-cell lymphoma in the modern treatment era. Br J Haematol. 2017;178:72–80.
22. Jung KW, Won YJ, Kong HJ, Lee ES; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–16.
23. IACR International Association of Cancer Registries. Korea Central Cancer Registry profile page [Internet]. Lyon: IACR Official Website; 2020. [cited 2018 Nov 1]. Available from: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=973&Itemid=498
24. Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–34.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||