AbstractPurpose5-Fluorouracil (5-Fu) is used as a conventional chemotherapy drug in chemotherapy for patients with advanced colorectal cancer, but many patients still suffer from treatment failure due to 5-Fu resistance. Emerging observations revealed the important role of chemokine (C-X-C motif) ligand 13 (CXCL-13) in tumor microenvironment and its relationship with prognosis in patients with colorectal cancer. This study is designed to reveal the important role of CXCL-13 in causing colorectal cancer resistance to 5-Fu.
Materials and MethodsCXCL-13 levels of patient's serum or cell culture supernatants were measured separately by enzyme-linked immunosorbent assay. In cell assays, cell viability is detected by Cell Counting Kit-8. Therefore, the recombinant human CXCL-13 was used to simulate its high expression in cells while its antibody and siRNA were used to reduce CXCL-13 expression in cells.
ResultsIn this study, we demonstrated that CXCL-13 is associated with 5-Fu resistance by culture medium exchange experiments and cytokine arrays of colorectal cancer resistant and non-resistant cells. Clinical studies showed that CXCL-13 is highly expressed in the serum of 5-Fu–resistant patients. High levels of serum CXCL-13 also predict a worse clinical outcome. The addition of recombinant CXCL-13 cytokine resulted in 5-Fu resistance, while its antibody overcame 5-Fu resistance, and knockdown of CXCL-13 expression by siRNA also reduced 5-Fu resistance, which can be saved by added recombination CXCL-13.
IntroductionAccording to 2018 global cancer data statistics, colorectal cancer (CRC) is the first cause of cancer-related death, ranking third in terms of incidence [1]. Despite the tremendous progress and numerous efforts in multidisciplinary therapy, survival and quality of life in patients with severe CRC are not optimistic [2]. The currently accepted treatment for CRC is FOLFOX or FOLFIRI treatment regimen consists of 5-fluorouracil (5-Fu)/leucovorin plus oxaliplatin or irinotecan with or without operative treatment [3]. As the basement of CRC chemotherapy, 5-Fu works by preventing DNA synthesis of CRC cells and inhibiting enzyme activity of thymidylate synthase. However, prolonged use of 5-Fu will cause tumors to be insensitive to drugs, and the patients with recurrent CRC are more likely to develop resistance to the original drug [4-6]. Therefore, it is urgent to reveal the molecular mechanism leading to drug resistance, which helps to find new prognostic biomarkers and overcome the drug resistance of tumors.
Some scientists have put forward hypothesis about 5-Fu resistance through research, including the abnormal activation of the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway and differential expression of certain cell cycle-associated proteins, miRNAs dysregulated [7], genes with promoter hypermethylation and concordant expression silencing [8] and so on. At the same time, more and more views support the crucial aspect of cytokines in the process of tumor cell resistance [9]. The strong regulation of cytokines in normal physiological processes and abnormal pathological processes, which more specifically including drug targeting, transport, and metabolic changes, is becoming more and more familiar to us [10,11]. So, it is difficult for scientists to believe that there is no close relationship between cytokines and tumor resistance.
Chemokine (C-X-C motif) ligand 13 (CXCL-13) is a member of small chemokines (8-10 kDa) [12], and it is a chemotactic protein targeting B lymphocytes, while its receptor, CXCR5, is expressed in specific T lymphocyte subsets [11]. In addition to the previous discovery that CXCL-13 is usually expressed by lymphoid organs, stromal cells and follicle dendritic cell, it also is secreted by some types of tumor cells, bone marrow endothelial cells, osteoblasts, myofibroblasts and CXCL-13–producing CD4+ follicular helper T cells [13-15]. So far, anecdotal evidence suggests that CXCL-13 together with CXCR5 might play increasingly vital roles in regulating tumor occurrence, progression, metastasis and prognosis in the microenvironment where the tumor is located [16-19]. Some recent studies have found that CXCL-13 has a predictive value for the prognosis of patients with CRC [20,21]. Besides those previous researches, the function of CXCL-13 in the response to CRC to 5-Fu–based chemotherapy and drug resistance remains a big mystery.
In this study, we used a cytokine array to detect cytokines in the conditioned medium (CM) of drug-resistant and non-resistant CRC cells respectively, and we were surprised to find some changes in cytokines. Our results suggest that CXCL-13 contributes to the CRC cells resistant to 5-Fu drugs in vitro. More importantly, this newly found mechanism is confirmed by our clinic evidence: 5-Fu drug–resistant patient was detected with higher level of CXCL-13 and there is a correlation between the higher level of CXCL-13 in the patient's serum and low overall survival (OS) rate or disease-free survival (DFS) rate of CRC patients receiving 5-Fu–based chemotherapy. Furthermore, we verified that the increased expression of CXCL-13 is closely related to the stimulation of 5-Fu by patient-derived xenografts (PDX) model. These findings indicate that CXCL-13 plays an important new role in the progression and chemosensitivity of CRC.
Materials and Methods1. Antibodies and reagentsThe recombinant human CXCL-13 was purchased from PeproTech (Rocky Hill, NJ). The antibody for CXCL-13 neutralization was from R&D Systems (Minneapolis, MN). 5-Fu was purchased from Sigma-Aldrich (St. Louis, MO).
2. Cell culture and chemicalsThe CRC cell line DLD-1 and HCT116 were bought from the American Type Culture Collection (Manassas, VA) and cultured respectively in medium of RPMI‑1640 (Gibco, Invitrogen, Carlsbad, CA) and Dulbecco's modified Eagle medium with higher glucose levels (Gibco, Invitrogen), containing 10% fetal bovine serum (Gibco, Invitrogen). All cells were cultured in a cell incubator at 37°C and 5% CO2 according to previous experiments. 5-Fu is diluted to a specific concentration by 10% dimethyl sulfoxide (Sigma-Aldrich).
3. Cell viability assayAfter 3 days of different concentrations of 5-Fu treatment, cell viability of DLD-1, DLD-1 5-FuR cells, and HCT116, HCT116 5-FuR cells was quantified by the Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Kyushu Island, Japan), as described manufacturers’ instructions. We used the 450 nm wavelength on the microplate reader to measure the absorbance. Three independent experiments in triplicate ensured repeatability of results.
4. Enzyme-linked immunosorbent assayCytokine analysis was performed on specific patient's serum or cell culture supernatants using human CXCL-13 enzyme-linked immunosorbent assay (ELISA) kits (Elabscience, Wuhan, China) according to the manufacturers’ instructions. Each sample was duplicated.
5. siRNA interferenceCXCL-13 siRNA and siRNA as control were purchased from Thermo Fisher Scientific (Carlsbad, CA). siRNA transfected cells by Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific) as described manufacturers’ instructions. Cells were treated with the indicated agents for analyses 24 or 48 hours after siRNA transfection.
6. ImmunohistochemistryFor paraffin tissue sections, after deparaffinized in xylene and hydrated with ethanol solutions, epitope retrieval was performed by boiling the de-paraffinized sections at 95-100°C for 20 minutes in Tris-EDTA buffer (pH 9.0). In order to quench endogenous peroxidase activity, slides were incubated with 3% hydrogen peroxide in methanol solution for 10 minutes. Nonspecific bindings were blocked by treating slides with blocking buffers which include some percentage of normal serum, non-fat dry milk, bovine serum albumin for 20 minutes. CXCL-13 1:20 antibody was co-incubated at 4°C for 1 hour and the second antibody was co-incubation at room temperature for 30 minutes. DAB staining was using DAB Kit (Shanghai Gene, Shanghai, China) according to the manufacturer’s instructions. Hematoxylin was used to counterstain the slides.
7. PatientsSerum samples were obtained from CRC patients from 2008 to 2018 and stored at the Key Laboratory of Biotherapy of Zhejiang province, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. The histological types, disease stages, and cancer cell contents in each case were examined by experienced pathologists. The treatment experienced by the patients included laparoscopic anterior resection, abdominoperineal resection and resection of the left or right colon followed by 5-Fu–based chemotherapy. The OS and the DFS were calculated strictly in accordance with the definition of World Health Organization. According to our definition of 5-Fu responses, 5-Fu–sensitive was described as patients who have a total response to 5-Fu–based therapy and no recurrence in 6 months, whereas 5-Fu–resistance means patients who had the recurrence occur in 6 months of completing 5-Fu–based therapy.
8. Animal experimentsFour weeks old female BALB-c-nu nude mice were purchased from SiBeiFu Biotechnology Co. Ltd. (Beijing, China). Patient-derived tumor xenograft tumors were imp-lanted subcutaneously in the groin on both sides of the nude mice. When the average tumor diameter reached 10 mm (about 2 weeks), the mice were randomized into subsequent experiment groups. Vehicle or 5-Fu (30 mg/kg) was given intraperitoneally weekly for 4 weeks. From the beginning of the medication, we take blood from the eyelids of nude mice every week to obtain serum. Tumor samples were obtained from the nude mice 3 days after the final medication time.
9. Statistical analysisGraph Pad Prism ver. 7.0 (Graph Pad, Inc., La Jolla, CA), SPSS ver. 22.0 (IBM Corp., Armonk, NY) and Image J 2.0 (National Institutes of Health, Bethesda, MD) were used to process and display data. Mean±standard deviation is the way we used to present data from three independent experiments tested in triplicates. The one-sample Kolmogorov–Smirnov test was used to test whether the experimental data were consistent with the normal distribution. If it were, we used the independent sample t test to analyze the data. If not, we used nonparametric test. The differences in survival curves were tested by the log-rank test or Cox regression. Data were analyzed by two-tailed analysis. p-values less than 0.05 were considered statistically significant.
10. Ethical statementFor the clinical specimens and animals used in the experiment, we passed the approval of the local ethics committee (IRB No. 20140213-19). All animal experiments were carried out in accordance with the guidelines for the ethical treatment of animals and with the approval of the Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University Animal Care and Use Committee.
Results1. CXCL-13 elevated in 5-Fu–resistant CRC cellWe obtained 5-Fu–resistant CRC cells by continuously increasing the concentration of 5-Fu in the culture solution [22]. Thence, starting with two 5-Fu–sensitive CRC cell lines (DLD-1 and HCT116 cells), we gained two sub-cell lines, called DLD-1 5-FuR and HCT116 5-FuR. Subsequent analysis of cell activity prompted that the 50% inhibitory concentration (IC50) of 5-Fu for DLD-1 5-FuR was increased by 13-fold and IC50 of 5-Fu for HCT116 5-FuR by 4-fold (Fig. 1A and B).
Since previous studies have pointed to a close relationship between tumor resistance and cytokines [9,23,24], we suspected that there are some special secreted factors in cell culture medium of DLD-1 5-FuR cells that lead to 5-Fu resistance. In order to verify this conjecture, we used DLD-1 5-FuR cells’ culture medium to culture DLD-1 cells and were surprised to find that, DLD-1 cells co-cultured in the CM from DLD-1 5-FuR showed stronger resistance to 5-Fu compared to DLD-1 cells cultured in the original medium (Fig. 1C), indicating that some secreted cytokines from DLD-1 5-FuR cells caused the 5-Fu resistance of DLD-1 cells. In addition, we repeated this experiment in HCT116 and HCT116 5-FuR cells and obtained similar results (Fig. 1D).
To determine which cytokines in the CM from DLD-1 5-FuR cells that result in CRC cells resist to 5-Fu, we used a cytokine array to measure the levels of various cytokines levels in the CM of DLD-1 and DLD-1 5-FuR cells. The result showed that some cytokines (Eotaxin, Fit-3 ligand, CXCL-13, IGFB1, etc.) in the CM of DLD-1 5-FuR cells changed dramatically, compared to its control cells. The semi-quantitative results of cytokines are shown in Fig. 1E. We found that CXCL-13 may be the most likely cytokine that causes CRC cells to resist to 5-Fu drug. Secreted CXCL-13 proteins levels by ELISA of our detection in sensitive and its resistant CRC cells make clear that the concentration of secreted CXCL-13 proteins significantly elevated in both resistant CRC cell lines than that cultured in supernatant of cell culture of non-resistant cell lines (Fig. 1F).
2. CXCL-13 is elevated in 5-Fu–resistant CRC patient and predicts clinical outcomesIn order to confirm and clarify the character of the CXCL-13 in 5-Fu–resistant CRC patients, the serum levels of CXCL-13 in patients of 5-Fu–sensitive (n=9) and 5-Fu–resistant (n=9) were assessed using ELISA. All patient information is detailed in Table 1. Compared with the patients of 5-Fu–sensitive (mean, 16.83 pg/mL), CXCL-13 was highly expressed in the patients of 5-Fu–resistant (mean, 268.97 pg/mL) (Fig. 2A). The mean expression level of CXCL-13 in 5-Fu–resistant patients was 16 times of that in 5-Fu–sensitive patients.
To evaluate the prognostic significance of the serum CXCL-13 levels, we tested the serum levels of CXCL-13 in a group of CRC patients and followed up their prognosis. Their information is detailed in Table 2. In all of the enrolled 64 patients with CRC, they received treatment according to the guidelines and completed more than 40 months of follow-up, most of which exceeded 60 months. We used serum CXCL-13 cut-off value 118 pg/mL to categorize patients as high expression of CXCL-13 (n=39) and low expression of CXCL-13 (n=25) groups. Our results indicate the expression level of CXCL-13 in serum is importantly related to OS and DFS of patients with CRC. More specifically, the group of high expression of CXCL-13 shows worse prognosis results from OS or DFS perspective than the low expression group (Fig. 2B and C). It is showed that there is a negative correlation between the expression of CXCL-13 in serum and the prognosis of patients with CRC after 5-Fu drug treatment.
3. CXCL-13-mediated autocrine mechanism promotes 5-Fu resistance in CRC cancer cellsThe above experimental results indicated that CXCL-13 might be a key cytokine contributing to CRC cell resistance to 5-Fu. So we designed gain of function experiments and loss of function experiments to prove our conjecture. We first tested whether the addition of recombinant CXCL-13 to the culture supernatant increased the 5-Fu resistance of DLD-1 and HCT116 cells. Indeed, when recombinant CXCL-13 was added to the culture supernatant of DLD-1 and HCT116 cells, the IC50 of the two cells is observably improved (Fig. 3A and B). Meanwhile, CXCL-13 neutralization antibody treatment decreased the IC50 of both DLD-1 5-FuR and HCT116 5-FuR cells, indicating that the sensitivity of cells to 5-Fu has increased (Fig. 3C and D). Thus, secreted CXCL-13 acts as a crucial autocrine factor to result in 5-Fu resistance in these CRC cells.
For further verification of the relationship between CXCL-13 and 5-Fu resistance in CRC cells, we knocked down CXCL-13 by siRNA in DLD-1 5-FuR and HCT116 5-FuR cells and found reduced expression of CXCL-13 in two drug-resistant cells (Fig. 4A). More importantly, knockdown of CXCL-13 re-sensitized DLD-1 5-FuR and HCT116 5-FuR cells to 5-Fu (Fig. 4B and C). With the increase of 5-Fu concentration in the cell culture medium, the greater the effect of CXCL-13 knockdown on CRC cell resistance of 5-Fu. After adding recombinant CXCL-13 to the cell culture medium, 5-Fu resistance of DLD-1 5-FuR and HCT116 5-FuR cells with downregulated CXCL-13 by siRNA had been significantly restored. The addition of recombinant CXCL-13 could regain resistance to 5-Fu in DLD-1 5-FuR and HCT116 5-FuR cells, the IC50 changes of the above cells were shown in Fig. 4D. These findings further proved that CXCL-13 is a major cytokine involved in CXCL-13–induced 5-Fu resistance in CRC cells.
4. 5-Fu treatment induces CXCL-13 secretion in vivoIn recent years, PDX models have developed translational medical research in the field of oncology for they retain the major histological and genetic features of their donor tumors. In a nutshell, it inoculates primary tumor tumors obtained from patients into immunodeficient mice. As a consequence, PDX models are widely used in biomarker development, drug evaluation, patient medication guidance, and metastasis study.
In order to further detect whether the tumor tissue will also cause an increase in CXCL-13 due to a similar response to 5-Fu in vivo, we constructed a PDX model with a tumor from a colon cancer patient who had not used 5-Fu ever, and subsequent chemotherapy showed that the tumor was sensitive to 5-Fu. When tumors reached a volume of ≥ 1,000 mm3 (about 2 weeks later), we treated xenograft tumor with a dose of 30 mg/kg twice a week via intraperitoneal injection for 4 weeks. After weekly drug treatment, the blood of the mice took around the eyelids was used to analyze the expression of cytokines. Seven days after the last treatment, tumor tissue is collected to analyze the expression of CXCL-13. The research process is briefly summarized in Fig. 5A.
There was no difference in serum CXCL-13 levels before starting 5-Fu injection. 5-Fu treatment significantly increased the expression of CXCL-13 in the serum of mice (Fig. 5B). Tumor tissues that had been subjected to 5-Fu strike exhibited higher levels of CXCL-13 expression by immunohisto-chemistry analysis (Fig. 5C). Quantitative immunohistochemical analysis confirmed our conclusion (Fig. 5D). Thus, sustained 5-Fu stimulation under in vivo conditions induces activation of CXCL-13 in tumor cells. Therefore, this in vivo experiment provides partial evidence for an explanation of how patients with CRC develop elevated CXCL-13 expression during 5-Fu chemotherapy.
DiscussionAccording to the article previously reported, cytokines, particularly those related to cell proliferation, migration, invasion, immune response, and tumor microenvironment, could partially explain the tolerance of tumor cells to chemotherapeutic drugs [9,23,25,26]. Therefore, we selected and cultured two 5-Fu–resistant CRC cell lines (DLD-1 5-FuR and HCT116 5-FuR), and confirmed that there are certain cytokines related to CRC cell resistance by exchanging the culture medium of drug-resistant and non-resistant cell lines. Further cytokine arrays led us to limit our research to CXCL-13. Through “gain of function” and “loss of function” experiments we confirmed the important role of CXCL-13 in CRC cell resistance to 5-Fu. Recent experimental results remind us that CXCL-13 siRNA reversed the DLD-1 5-FuR and HCT116 5-FuR cell resistance to 5-Fu drug, while adding recombinant CXCL-13 could restore these cell resistance. However, which cell pathways CXCL-13 participates in the CRC cells resist to 5-Fu still requires further experimental exploration.
CXCL-13, which expressed in lymphoid organs and some tumor tissues, has attracted a lot of attention for its important role in attracting tumor cells spreading to these sites and promoting bad clinical outcomes [17,27,28]. Zhu et al. [29] study showed CXCL-13 promoted colon cancer cells growth and migration via activating the PI3K/AKT pathway. Furthermore, CXCL-13 promoted matrix metalloproteinase 13 expression and secretion. Qi et al. [27] research results showed the expression of CXCL-13 and CXCR5 were noticeably increased in CRC tissues compared with adjacent cancer cells, which was more apparent in tumors with higher tumor staging (≥ T3). However, Waldner et al. [30] found the number and the size of intestinal tumors in CXCR5-knockout mice were noticeably reduced, and the number of infiltrated B cells in the tumor was found to increase by a large margin compared with that of control group. In contrast, mice treated with CXCL-13-overexpressing MC38 cells showed lower tumor growth rates and sizes. It seems that CXCL-13-CXCR5 axis may target tumor by recruiting B lymphocytes, and is essential in the anti-tumor immune response of tumor microenvironment in CRC. The above evidence suggests that CXCL-13 may be a potential target in CRC therapy.
What counts is this new molecular mechanism of CRC not sensitive to 5-Fu was verified by our clinical findings. For the detection of CXCL-13 in the serum of patients who are not sensitive to 5-Fu, its content is much higher than that of the counterpart. This result is consistent with the study's conclusion of Qi et al. [27]. Thus, patients with elevated CXCL-13 are more likely to show resistance to 5-Fu and patients with this feature suggest a worse prognosis (including OS and DFS) following 5-Fu–based therapy. Although we have controlled the patients’ age, sex, underlying condition, and pathological type of the tumor, there are still some differences in the patient's baseline. As we collect more cases for analysis in the future, we will further balance the situation between the groups and try to discover the relationship between CXCL-13 and other clinical features.
As a hotspot tool for tumor research in recent years, PDX model also plays an important role in the study of tumor resistance. In this study, we used the PDX model to reconstruct the in vivo performance of tumors after 5-Fu treatment. Consistent with the cell experiment, the tumor showed a higher level of CXCL-13 under continuous 5-Fu treatment, both in serum and in tissue. The above experimental results strongly demonstrate that the high expression of CXCL-13 is closely related to tumors stimulated by 5-Fu. In subsequent experiments, the PDX model could also be used to explore a range of mechanisms and potential therapeutic targets for CXCL-13 leading to tumor resistance.
The discovery of CXCL-13 autocrine mechanism induces tumor resistance to 5-Fu is an exciting thing. Since 5-Fu treatment stimulates the secretion of CXCL-13, then we have more reasons to believe that joint use of a 5-Fu drug and an agent targeting CXCL-13 could prevent or delay the emergence of CRC cell resistance to the 5-Fu during the during chemotherapy. Perhaps we can detect the level of CXCL-13 in the serum of chemotherapy patients, find patients with 5-Fu resistance as soon as possible, and take active treatment measures. However, our hypothesis must be confirmed by more experimental results and clinical trials.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (No.81771502, 81701820), the Natural Science Foundation of Zhejiang Province (No. LH19H160001) and the Department of Health of Zhejiang Province (No. 2018KY473 and 2018PY-0250). This article represents partial fulfillment of the requirements for a Master degree for Guolin Zhang.
Fig. 1.Chemokine (C-X-C motif) ligand 13 (CXCL-13) is elevated in 5-fluorouracil (5-Fu) resistant (5-FuR) colorectal cancer cells. (A, B) Viability of DLD-1 and DLD-1 5-FuR cells, HCT116, and HCT116 5-FuR cells treated with different concentrations of 5-Fu for 3 days. (C, D) Viability of DLD-1 cells or HCT116 cells treated with different concentrations of 5-Fu for 3 days after pretreatment with DLD-1 5-FuR or HCT116 5-FuR cells conditioned medium (CM) for 2 days. (E) Semi-quantitative of cytokine arrays analysis of expression levels of different cytokines measured in the CM of paired DLD-1 and DLD-1 5-FuR cells. (F, G) Box-plot showing the CXCL-13 expression level in colorectal cancer parental cells (DLD-1, HCT116) and 5-FuR cells (DLD-1 5-FuR, HCT116 5-FuR). Data from three independent experiments were tested in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test. 
Fig. 2.Chemokine (C-X-C motif) ligand 13 (CXCL-13) is elevated in 5-fluorouracil (5-Fu) resistant colorectal cancer patient and predicts clinical outcomes. (A) Comparison of the levels of CXCL-13 protein were measured by enzyme-linked immunosorbent assay in 5-Fu–sensitive (n=9) and –resistant (n=9) colorectal cancer patients, patients are shown in Table 1. Sen, sensitive cases; Res, resistant cases. (B) 6-Year overall survival (OS) Kaplan–Meier survival curves grouped by serum CXCL-13 expression levels (118 pg/mL) of 64 colorectal cancer patients, as patient information is shown in Table 2. (C) Six-year disease-free survival (DFS) Kaplan–Meier survival curves grouped by serum CXCL-13 expression levels (118 pg/mL) of 64 colorectal cancer patients, as patient information is shown in Table 2. (A) *p < 0.05, **p < 0.01 by unpaired Student’s t test. (B, C) *p < 0.05 by log-rank (Mantel-Cox), hazard ratios (HRs) and 95% confidence interval (CI) are shown. 
Fig. 3.Chemokine (C-X-C motif) ligand 13 (CXCL-13) promotes colorectal cancer cells resistance to 5-fluorouracil (5-Fu) through autocrine mechanism. (A, B) Viability of DLD-1 cells or HCT116 cells treated with different concentrations of 5-Fu for 3 days after pretreatment with 10 ng/mL of CXCL-13 for 6 hours. (C, D) Viability of DLD-1 5-Fu resistant (5-FuR) cells or HCT116 5-FuR cells treated with different concentrations of 5-Fu for 3 days after pretreatment with a control IgG or a CXCL-13 neutralizing antibody (5 μg/mL) for 6 hours. Data from three independent experiments were tested in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test or one-way ANOVA. 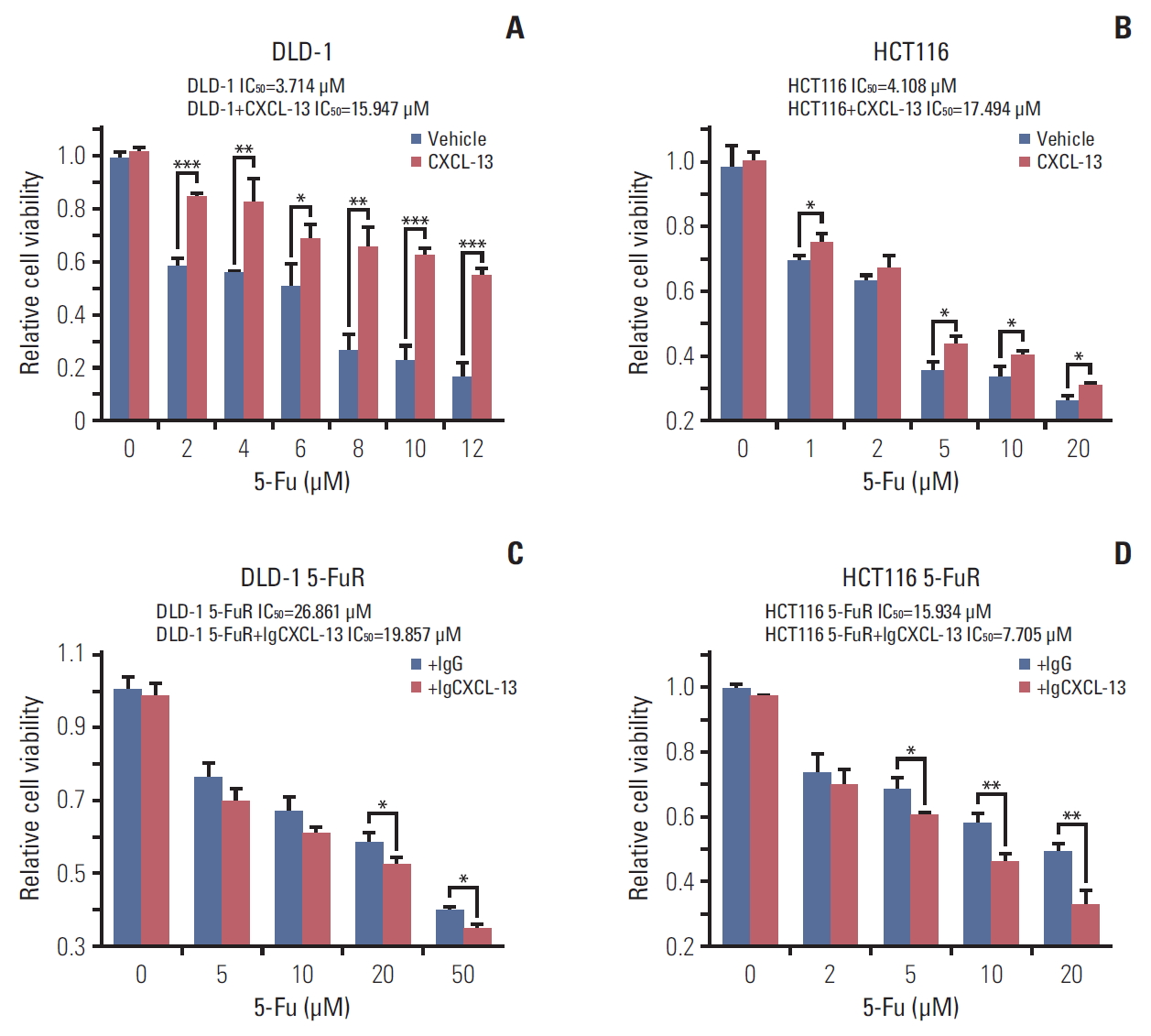
Fig. 4.Knockdown of chemokine (C-X-C motif) ligand 13 (CXCL-13) overcomes 5-fluorouracil (5-Fu) resistance (5-FuR) in colorectal cancer cells. (A) Box-plot showing the CXCL-13 expression level in DLD-1 5-FuR cells or HCT116 5-FuR cells, DLD-1 5-FuR or HCT116 5-FuR siCXCL-13 cells (30 pg/mL for siCXCL-13). (B, C) Viability of DLD-1 5-FuR cells or HCT116 5-FuR cells, DLD-1 5-FuR or HCT116 5-FuR siCXCL-13 cells (30 pg/mL for siCXCL-13) and recombinant CXCL-13 (10 ng/mL) reversed the effect of CXCL-13 siRNA on the sensitivity of DLD-1 5-FuR cells or HCT116 5-FuR cells treated with different concentrations of 5-Fu for 3 days after pretreatment with CXCL-13 siRNA. (D) The 5-Fu concentration of 50% inhibition of cell growth (IC50) of six cells in B and C above. Data from three independent experiments were tested in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test or one-way ANOVA. 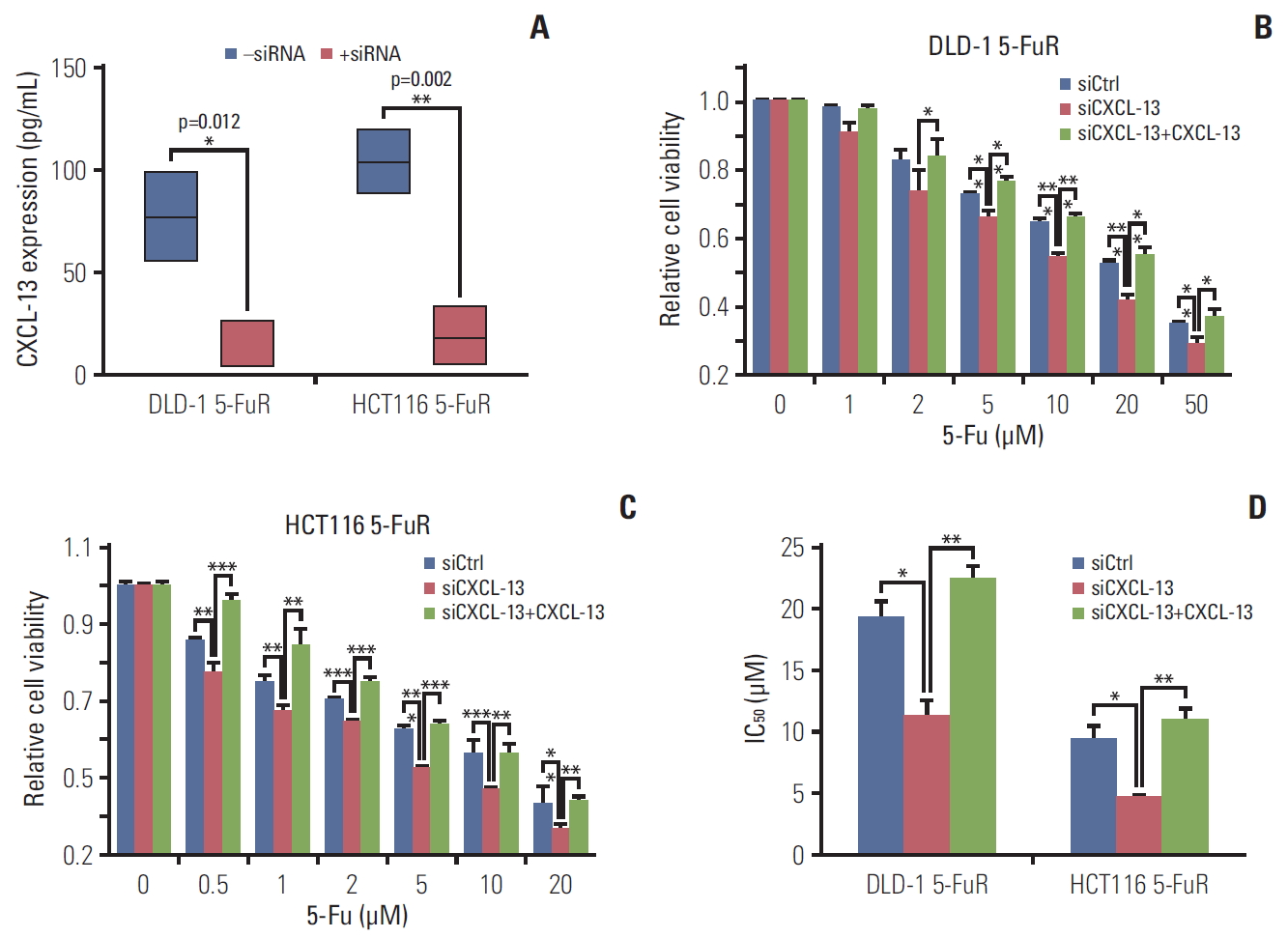
Fig. 5.5-Fluorouracil (5-Fu) treatment induces chemokine (C-X-C motif) ligand 13 (CXCL-13) secretion in vivo. (A) A simple schematic of the experimental process. (B) Line chart showing the serum CXCL-13 expression level in mice bearing patient-derived tumor xenograft (PDX) tumors and treated with vehicle or 5-Fu (30 mg/kg/twice a week for 4 weeks) as in A. (C) Representative H&E, immunohistochemistry (IHC) images of subcutaneous tumors formed by PDX tumors treated as in A. (D) Quantification of IHC staining for CXCL-13 of subcutaneous tumors formed by PDX tumors treated as in A. ELISA, enzyme-linked immunosorbent assay. Data are represented as mean±standard deviation. n=3 mice/group while n=6 tumors/group. **p < 0.01 by Student’s t test or two-way ANOVA. 
Table 1.Patient characteristics of 5-Fu–sensitive or –resistant set Table 2.Correlation between patient serum CXCL-13 level and clinical characteristics of ELISA set References1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
2. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78.
3. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14.
4. Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014;20:9775–827.
5. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–66.
6. Iyer AK, Singh A, Ganta S, Amiji MM. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv Drug Deliv Rev. 2013;65:1784–802.
7. Xie T, Huang M, Wang Y, Wang L, Chen C, Chu X. MicroRNAs as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell Physiol Biochem. 2016;40:62–76.
8. Shen Y, Tong M, Liang Q, Guo Y, Sun HQ, Zheng W, et al. Epigenomics alternations and dynamic transcriptional changes in responses to 5-fluorouracil stimulation reveal mechanisms of acquired drug resistance of colorectal cancer cells. Pharmacogenomics J. 2018;18:23–8.
9. Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta. 2016;1865:255–65.
10. Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28.
13. Huber AK, Irani DN. Targeting CXCL13 during neuroinflammation. Adv Neuroimmune Biol. 2015;6:1–8.
14. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–92.
15. Chen L, Huang Z, Yao G, Lyu X, Li J, Hu X, et al. The expression of CXCL13 and its relation to unfavorable clinical characteristics in young breast cancer. J Transl Med. 2015;13:168.
16. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2:91487.
17. Fan L, Zhu Q, Liu L, Zhu C, Huang H, Lu S, et al. CXCL13 is androgen-responsive and involved in androgen induced prostate cancer cell migration and invasion. Oncotarget. 2017;8:53244–61.
18. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–44.
19. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95.
20. Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, et al. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560–7.
21. Olsen RS, Nijm J, Andersson RE, Dimberg J, Wagsater D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23:6212–9.
22. Murakami Y, Kazuno H, Emura T, Tsujimoto H, Suzuki N, Fukushima M. Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int J Oncol. 2000;17:277–83.
23. Butera G, Pacchiana R, Donadelli M. Autocrine mechanisms of cancer chemoresistance. Semin Cell Dev Biol. 2018;78:3–12.
24. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51.
25. Miller MA, Sullivan RJ, Lauffenburger DA. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin Cancer Res. 2017;23:623–9.
26. Zhou W, Sun W, Yung MMH, Dai S, Cai Y, Chen CW, et al. Autocrine activation of JAK2 by IL-11 promotes platinum drug resistance. Oncogene. 2018;37:3981–97.
27. Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, et al. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18:1916–24.
28. Lillard J, Singh R, Sharma P, Singh S. CXCL13 inhibition prevents bone metastasis in hormone-refractory prostate cancer (133.8). J Immunol. 2010;184:133.8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||