INTRODUCTION
Radiation myelopathy is a rare late complication following curative radiotherapy. Yet once this damage has occurred, this injury leads to a fatal condition; thus, radiation myelopathy is the principal dose limiting factor of radiotherapy and it causes most radiotherapists to limit the total dose, which compromises local tumor control (1). White-matter necrosis and demyelination are the typical manifestations of radiation myelopathy. The pathogenesis is thought to include damage to the glial progenitors and the vasculature, and this induces functional changes through the impaired blood flow (2). There is great recent interest in the potential value of pentoxifylline (PTX) as an inhibitor of the normal tissue injury caused by radiation (3). PTX is a methylxanthine derivative that produces dose-related hemorrheologic effects like lowering blood viscosity, increasing red blood cell deformity, inhibiting platelet aggregation, stimulating release of prostacyclin and increasing tissue oxygen levels. It was first delivered to patients who suffered from vascular disease to improve their tissue perfusion. It also has a phytochemical antioxidant effect that acts as a radical scavenger and it may regulate the expression of TNF-α and the other inflammatory cytokines involved in cell signaling (4). Many promising early laboratory and clinical investigations have already showed the possibility that PTX can be used to limit vascular disturbances and so reduce the late radiation damage (3~5).
We previously observed that PTX might slightly increase the isoeffect dose required to induce leg paresis for fractionated irradiation, although this finding was not statistically significant (5). Thereafter, we conducted an experiment with using a single fraction (i.e.: large fraction size) to get a DMF of 1.14, which was the greatest value we had ever achieved. Based on the possibility that the effect of PTX may be dependent on the fraction size, we tried to elucidate the radiobiological parameters (α/β, T1/2) that are modified by PTX.
MATERIALS AND METHODS
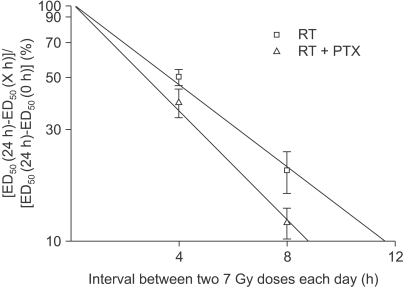
Adult female Sprague-Dawley rats (7 weeks old, median weight: 250 g) were used in this study. Two study groups were defined. The experimental group was treated with PTX in drinking water starting at 1 week prior to radiation, and this continued through 40 weeks after the radiation treatment. The control group received sterile drinking water without PTX. Pentoxifylline (PTX, Handok pharmaceutical Co., Seoul, Korea) was added to the distilled drinking water at a concentration of 2 g/L; the drinking water was consumed ad libitum. A 2 cm segment of the cervical spinal cord (C1 through T2) was irradiated with a 6MV LINAC at a dose rate of 3 Gy/min. The animals were irradiated with single, two, four and eight fractions with a fraction interval of 24 h. Each fractionation scheme was comprised four or five dose levels and there were 6 animals used for each dose level. For the control animals, the total doses ranged from 13 to 23 Gy, 27 to 37 Gy, 36 to 48 Gy and 44 to 54 Gy for the single, two, four and eight fractions, respectively. The corresponding total doses for the PTX groups ranged from 16 to 27 Gy, 30 to 41 Gy, 40 to 53 Gy and 48 to 59 Gy, respectively. The endpoint of the experiment was leg paresis and follow-up of the animals was continued for up to 40 weeks after the last irradiation. If we assume that the observed paralytic effects of cervical irradiation are a consequence of equivalent levels of cell lethality (for example, this was chosen for illustrative purposes to be equal to e-1), then α/β can be calculated via reciprocal-dose analysis (6). After tabulation of the ED50 values (the estimated dose needed to produce 50% paralysis in a group of irradiated animals) with using the Litchfield and Wilcoxon method (7), plotting the reciprocal of each ED50 against the corresponding dose per fraction (=ED50/n) and performing linear least square regression resulted in a straight line. The intercept on the ordinate gave α, while the slope gave β. Subsequently, to obtain the relevant data on repair kinetics, the animals in the experimental group received a pair of 7 Gy fractions on each day, separated by intervals of 4 and 8 h. The repair half time was analyzed by the traditional process (8). The ED50 values were plotted linearly against the interval between the two 7 Gy doses on each day to show the pattern of repair. The ED50 at the 24 h interval (7 Gy-24 h-7 Gy) was taken as corresponding to complete 100% repair and the ED50 at the 0 h interval (7 Gy-0 h-7 Gy) as corresponding to zero repair. Making the semilog plot required an estimate of ED50 for the 24 h interval, that is, with a 7 Gy dose given daily and the ED50 for the zero interval, that is, with a 14 Gy dose given daily. This dose-response curve was not obtained from the present set of experiments and these ED50 values were extrapolated from the existing data (Fig. 1) with assuming that 7 or 14 Gy fractions had been given daily instead of the doses that were actually used. From this, the estimated ED50 for the 7 and 14 Gy were 57.1 and 34.6 Gy for RT alone, respectively, and 63.3 Gy and 40.3 Gy for RT+PTX. The difference between the ED50 at any other time interval and the ED50 at 24 h was taken as a measure of the incomplete repair and this was plotted logarithmically as a percentage of the difference between the 24 h and the zero time ED50 against the linear time interval. The inverse slope (ln2/slope) of the resulting regression line would give the T1/2 if the incomplete-repair model with monoexponential kinetics was appropriate (8). The detailed description of the materials and methods we used has been reported in our previous paper (5). The significance of differences was tested using the t-test.
RESULTS
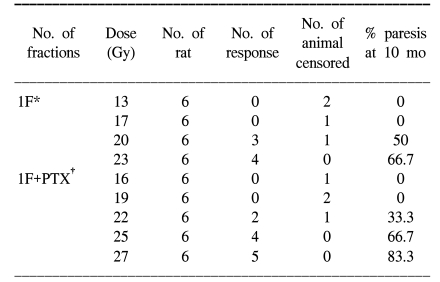
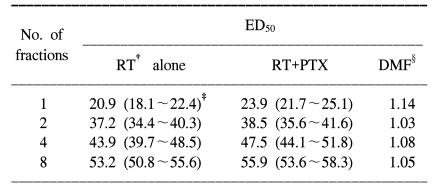
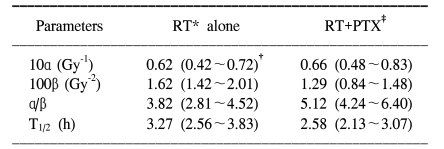
For all the schedules of the RT alone and RT+PTX groups, paresis developed in most of the animals after latent periods of 4 to 5 months and within the 7 month period after the radiation treatment. At up to 48 weeks follow-up (12 months), there is no additional occurrence of paralysis after 7 months. The latency was not influenced by the administration of PTX. The mean latent times were 172 days (median: 175 days) and 178 days (median: 180 days) for the RT alone group and the RT+PTX group, respectively. Therefore, all the data were analyzed using the quantal response without any latent-time correction. In our previous paper, the raw data of the fractionated group was described in detail (5) and the incidence of myelopathy and of censoring with a single fraction are summarized in Table 1. The ED50 values calculated from the probit curves are showed in Table 2 with their 95% confidence limits. As can be seen, the ED50 increases with the decreasing fraction size when daily fractions were given. The ED50 values for single, two, four and eight fractions for the RT alone group were 20.9, 37.2 43.9 and 53.2 Gy, respectively. The corresponding ED50 values for the RT+PTX group were 23.9, 38.5, 47.5 and 55.9 Gy, respectively. It was found that PTX has a tendency to increase the isoeffect dose required to induce leg paresis for all the fractionation schedules, but this was not statistically significant (p>0.05). Dose modification factors (DMF; the ratio of ED50 for RT alone to that for RT+PTX) of 1.14, 1.03, 1.08 and 1.05 were obtained for the single, two, four and eight fractions, respectively. To estimate α/β of the linear quadratic model, the paresis data was analyzed using reciprocal-dose plots. The results of these calculations are summarized in Table 3 and the regression lines are presented in Fig. 1. The α values calculated for RT alone and RT+PTX were almost the same (0.62 vs 0.66, respectively). It was noticeable that the β value (1.29) for the RT+PTX was lower than that (1.62) for RT alone. So, the α/β ratio (5.12) for the RT+PTX is higher than that for the RT alone (3.82), indicating that the capacity of SLD repair is changed with administration of PTX. However, the confidence intervals showed that the differences were not significant. To estimate the repair half time change induced by PTX, we drew the semilogarithmic plot of the ED50 values, which were expressed as percentages of the difference between an estimated ED50 for complete repair and the ED50 for zero repair, as a function of the interval between the two 7 Gy fractions per day (Fig. 2). The T1/2 obtained from the monoexponential model was 3.27 and 2.58 h for RT alone and RT+PTX, respectively. PTX expedited the repair process and decreased the repair half time, but these differences were not statistically significant (p>0.05)(Table 3).
DISCUSSION
We previously had a clue that PTX could protect the spinal cord from damage induced by fractionated irradiation, although this was not statistically meaningful (5). The DMF ranged from 1.03 to 1.08. Afterward, an experiment using a single fraction (i.e.: a large fraction size) was carried out to get a DMF of 1.14, which encouraged us to explore the radiobiological parameters (α/β, T1/2) that are potentially modified by PTX. α and β are the non-repairable and repairable parameters, respectively, of the linear quadratic model. α is a measure of the steepness of the initial linear segment of the dose-response curve (6,9). Accordingly, at doses per fraction of 1~2 Gy, the observed effect increases with α, on the contrary, β governs the bending region of the curve with a higher dose. From the reciprocal-dose analysis, the α values for RT alone and RT+PTX were almost the same, but the β value (1.29) for the RT+PTX was lower than that (1.62) for RT alone. So, the α/β ratio (5.12) for the RT+PTX was higher than that for RT alone (3.82). This means that PTX, which distinctly acts upon the curvier region of the response curve, may therefore be expected to make surviving cells more radioresistant to a higher dose. Bentzen et al correlated an increase in the α/β ratio with decreased fractionation sensitivity (10). The α/β ratio is a measure of the response of the system to changes in the fraction size. A large value of α/β in which α>>β would imply a small sensitivity to changes in fractionation. By contrast, a smaller value of α/β would imply a larger sensitivity to changes in fractionation. Consequently, the value of the α/β ratio, sometimes called the repair capacity, is a key determinant of fractionation sensitivity. The PTX decreased the fractionation sensitivity (an increased α/β ratio) of the rat spinal cord. Therefore, PTX may differently affect the tolerance of the spinal cord depending on the fraction size. PTX increased the tolerance more significantly in hypofractionation (a large fraction size) than in hyperfractionation (a small fraction size). Clinically, in case of using a hypofractionation scheme like stereotactic radiosurgery, PTX may have a more protective role for the spinal cord.
PTX induced β (the constant of the quadratic component) changes; this suggested that in the rat spinal cord, one of the mechanisms of radioprotection was an enhancement of cellular repair of radiation-induced sublethal lesions and a decreased repair half time T1/2. The chosen interval of 4 and 8 h between the two fractions given on one day was assumed to be a interim between complete (24 h) and zero (0 h) repair, according to the reported T1/2 of 2~4 h for rat spinal cord (11~13), and the difference in ED50 values after 1 and 2 fractions per day is supposed to be the result of incomplete repair. The calculated T1/2 was 3.27 and 2.58 h for RT alone and RT+PTX, respectively, when monoexponential decay of the sublethal damage (SLD) is assumed. PTX facilitated the repair process to make the repair half time shorter. It has been recently suggested that repair in the spinal cord may exhibit two decay components (fast and slow) of the SLD. Hopewell et al suggested the T1/2 values of 10.6 min and 2.4 h for the spinal cord on the basis of graphical analysis (12). Ang et al have reported T1/2 values of 0.7 and 4 h for the rat spinal cord, with 65% of the damage repaired by the long repair T1/2, when the experimental data was fitted according to a biexponential repair model (13). In this study, the number of data points was not sufficient for assessing the potential influence of PTX on the kinetics of the fast repair process. Nevertheless, the data suggested the presence of a slow repair component and it clearly showed significant continued recovery up to 24 h. Anyway, further investigation is required on the sophisticated nature of repair kinetics because the early effect of PTX on the radiation-induced cytokine and gene expression has also been proposed to be involved in the process of repair (14). In general, the decay of SLD is both dose and interval dependent, and it shows repair saturation as time passes by, indicating that the repair process requires various substrates and nutrients. It is also well known that the repair process is dependent on oxygen as it is an energy demanding procedure (15). Thus, the main mechanism of radioprotection (repair) caused by PTX may come about from the increased blood flow to speed up delivery of substrates and oxygen. Actually, Thompson et al showed that PTX significantly improves the spinal cord blood flow in experimental spinal cord injury (16). It's very interesting that when PTX was added 30 min after irradiation, a decreased number of DNA strand breaks was detected by alkaline comet assay (17). This would suggest that drugs do not simply act as radical scavengers that interfere with the initial events, but rather, they could act in a significant manner with the secondary events (the repair process) that occur during the first hours after irradiation.
PTX has been reported to prevent radiation-induced cell cycle arrest at the G2/M checkpoint of cancer cells, where damage repair is critically linked to cell survival. Danielsson et al reported that the enhanced cytotoxic effect of PTX was statistically significant in three squamous cell carcinoma cell lines, FaDu, RPMI 2,650 and SCC-61 (18). Eley et al also showed that PTX exerted a favorable radiosensitization effect in the human glioma cell line by abrogating the radiation-induced G2/M block and diminishing the DNA damage repair at this checkpoint (19). Additionally, Theron et al have insisted that radiosensitization by pentoxifylline is not a consequence of G2-block abrogation alone, but that direct inhibition of DSB repair plays a role in certain cancer cell types (20). For linacbased stereotactic radiosurgery, treatment is delivered intermittently via multiple individual small radiotherapy arcs. The time intervals between the individual arcs provide greater damage repair and increased tumor cell survival. So, a therapeutic gain could be achieved with combined PTX and radiation therapy, especially with using the hypofractionation regimens that are employed for stereotactic radiosurgery. Nevertheless, in this study, the DMF for the radiation myelopathy was a little bit smaller than that for the other normal tissues. This might include differences in the rate and extent of cellular uptake and the conversion of the drug to the active metabolite. So, escalation of the PTX dose, by means of an inthathecal or intravenous route of administration, should be considered before conducting clinical trials.
CONCLUSIONS
PTX increased the α/β ratio and it decreased the T1/2 of radiation myelopathy, suggesting that a decreasing fractionation sensitivity occurred. This implies that PTX, which distinctly acts upon the bending region of the high dose, may be expected to protect the spinal cord with a larger fraction size.