AbstractPurposeWe investigated the clinical relevance and spectrum of BRCA1/2 mutations in Korean ovarian cancer (KoOC) patients.
Materials and MethodsTwo hundred seventy-nine KoOC patients were enrolled from three university hospitals between 2012 and 2017. Their peripheral blood samples were obtained for BRCA1/2 mutation analysis by direct sequencing. Clinicopathological characteristics were retrospectively reviewed, and spectrum analyses of BRCA1/2 mutation were assessed by systematic literature review.
ResultsFrequency of BRCA1/2 mutations was 16.5% in KoOC patients. BRCA1/2 mutations were significantly associated with family history of breast/ovarian cancer (p<0.001), serous histology (p=0.044), and advanced International Federation of Gynecology and Obstetrics (FIGO) stage (III/IV, p=0.018) but not with early age-of-onset (age < 50, p=0.729). Literature review of BRCA1/2 mutations in KoOC patients found 111 (55 distinct) mutations with high proportion of Korean-specific mutations (24/55, 43.6%). Comparing the spectrum of BRCA1/2 mutation between KoOC and Korean breast cancer (KoBC) patients, the ratio of BRCA1-to-BRCA2 mutations was different, with BRCA1 (78.4%) being predominant in KoOC and BRCA2 in KoBC (59.2%). The most common mutation also differed between the two (c.3627insA of BRCA1 in KoOC and c.7480C>T of BRCA2 in KoBC).
ConclusionThe clinical relevance of BRCA1/2 mutations in KoOC patients was confirmed but that of early age-of-onset was not. Possible inconsistency in the ratio of BRCA1-to-BRCA2 mutations and the most common mutation between KoOC and KoBC may probably suggest presence of mutation sequence-associated penetrance tendency in hereditary Korean breast and ovarian cancer. These data may provide insights for optimal genetic counseling and prophylactic treatment for at-risk relatives of KoOC patients.
IntroductionEpithelial ovarian cancer (EOC) is the leading cause of cancer-related deaths in women worldwide and remains the most lethal gynecological cancer [1]. Although the incidence of EOC has traditionally been lower in Korea than in western countries, including the United States and United Kingdom, it has steadily increased over the last few decades because of lifestyle and environmental changes [2]. In 1999, the age-standardized rates for annual EOC incidence and mortality in Korea were 5.0 and 1.7 per 100,000 women-years, respectively, which significantly increased to 6.4 and 2.3 per 100,000 women-years, respectively, by 2014 (both, p < 0.05) [3]. Such increases represent a significant challenge to overcome, and methods for controlling the increasing rates of this cancer are urgently needed.
Defects in BRCA1/2 tumor suppressor genes are the most frequently identified genetic changes associated with ovarian cancer. A BRCA1/2 germline mutation is associated with poor prognostic factors, including early-onset cancer, serous histology, and advanced cancer stages [4]. However, in recent years, accumulating evidence has shown that BRCA1/2 mutations are associated with improved survival and improved response to not only platinum-based chemotherapy [5,6] but also targeted therapy using poly(ADP-ribose) polymerase inhibitors [7,8].
Although there have been several reports of BRCA1/2 mutation analyses in ovarian and breast cancer cases from Asian populations, there is relatively limited data for the Korean ovarian cancer (KoOC) population. Until now, there have been four independent studies on BRCA1/2 mutations in KoOC patients; however, all of these were relatively small-scale, single-center studies with marked inter-study heterogeneity among their results [9-11]. Furthermore, there has been no spectrum study of BRCA1/2 mutations in KoOC patients, such as nation-wide the Korean Hereditary Breast Cancer (KOHBRA) study [12,13]; KOHBRA study included a total of 2,953 breast cancer patients with high risk of hereditary breast and ovarian cancer (HBOC) enrolled from 36 institutions between 2007 and 2013, covering approximately 2.9% of the total Korean breast cancer (KoBC) patients during the study period. Thus, we performed comprehensive analyses to estimate the prevalence, clinicopathological significance, spectrum, and penetrance of BRCA1/2 mutations in a large cohort of KoOC patients.
Materials and Methods1. PatientsA total of 279 EOC patients unselected for family history (FH) were enrolled between May 2012 and December 2017 from three investigational sites in Korea: the Pusan National University Hospital, Inje University Paik Hospital, and Kosin University Gospel Hospital (PIKO). Genetic testing of BRCA1/2 genes in all three investigational hospitals was carried out by the Green Cross Company (Yongin, Korea) during the study period. The inclusion criteria for enrollment were primary EOC patients who had undergone staging surgery and had pathologically confirmed with EOC. Clinical and pathological data were collected for all patients: age at diagnosis, EOC grade, lymph node (LN) metastasis, FIGO stage (International Federation of Gynecology and Obstetrics, 2013), and personal history and FH of cancer. Patients with FH were defined as those with primary breast cancer or with first- or second-degree relatives with breast or ovarian cancer. FH of other cancer include all type of hematologic and non-hematologic malignancies.
2. Direct sequencingGenomic DNA was obtained from peripheral blood leukocytes and was extracted using the Promega DNA purification kit (catalog No. LA1620, Madison, WI) according to the manufacturer’s instructions. All exon and intron boundaries of BRCA1/2 were analyzed for mutations using a combination of denaturing high-performance liquid chromatography (WAVE DNA Fragment Analysis System, Transgenomic Inc., San Jose, CA) and bi-directional sequencing with a Big Dye Terminator V3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, CA) and ABI 3500xl (Life Technologies).
3. Statistical analysesThe Human Genome Variation Society nomenclature system (http://www.hgvs.org/mutnomen) was used for describing sequence variations, and the Breast Cancer Information Core (BIC) database (http://research.nhgri.nih.gov/projects/bic/Member/index.shtml) was used for evaluating results. Relationships among categorical variables were evaluated using t or chi-square tests. For all analyses, a p-value < 0.05 was considered statistically significant. Analyses were performed using SPSS ver. 20.0 (IBM Corp., Armonk, NY).
Results1. Prevalence of BRCA1/2 mutationsIn our direct sequencing analysis of BRCA1/2 genes in the 279 unselected EOC patients, pathogenic BRCA1 and BRCA2 mutations were observed in 35 (12.6%) and 11 (3.9%) cases, respectively. The overall prevalence of BRCA1/2 mutations in KoOC patients was 16.5% (Fig. 1A). Among the 279 EOC patients, 60 patients (21.5%) had FH of breast or ovarian cancer and 219 (78.5%) did not. The prevalence of BRCA1/2 mutations was higher in the former (21 of 60, 35.0%) than in the latter (27 of 219, 12.3%) (Fig. 1B). In specific subgroups according to tumor histology (serous vs. non-serous) and FIGO stage (I/II vs. III/IV), the prevalence of BRCA1/2 mutations was 11.9 % in patients with early stage (I/II) and serous histology, 23.1% in patients with advanced stage (III/IV) and serous histology, 0% in patients with early stage (I/II) and non-serous histology and 16.7% in patients with advanced stage (III/IV) and non-serous histology (S1 Fig.).
2. Clinical characteristics of BRCA1/2 mutationsRelationships between clinicopathological characteristics and BRCA1/2 mutations are listed in Table 1. The mean ages at the time of onset for patients with and without BRCA1/2 mutations were 53.7±9.6 years and 54.2±10.9 years, respectively, with no significant difference between them (p=0.765). In addition, we found no significant difference in the proportion of patients with early age-of-onset (< 50 years) of ovarian cancers between BRCA1/2mutation carriers and non-carriers (39.1% vs. 30.5%, p=0.223). Patients with BRCA1/2mutations exhibited significantly higher rates of FH of breast or ovarian cancer (45.7%) and other cancers (39.1%) compared with those without BRCA1/2 mutations (16.7% for FH of breast or ovarian cancer; 18.5% for FH of other cancers; both, p < 0.001). The occurrence of serous histology was significantly higher in patients with BRCA1/2 mutations (91.3%) than in those without BRCA1/2 mutations (68.7%, p=0.002). Four patients with BRCA1/2 mutations (8.7%) were identified as having non-serous histology in this study, with three cases of clear cell-type histology with BRCA1 mutations and one case of endometrioid-type histology with a BRCA2mutation (S2 Table). In addition, patients with BRCA1/2 mutations were more likely to be at an advanced FIGO stage of III/IV (87.0%) and exhibit LN metastasis (48.8%) than those without BRCA1/2 mutations (61.4%, p=0.007 for advanced FIGO stage of III/IV; 28.8%, p=0.011 for LN metastasis) (Table 1). However, multivariate analysis using logistic regression identified FH of breast/ovarian cancer (p < 0.001), serous histology (p=0.044), and advanced FIGO stage (III/IV, p=0.018) as the only factors showing independent correlation with BRCA1/2 mutations (p < 0.05) (Table 2).
3. Spectrum of BRCA1/2mutationsAll 46 pathogenic BRCA1/2 mutations detected in this study are listed and summarized in S2 Table. Thirty-two distinct mutations were identified, including 21 in BRCA1 and 11 in BRCA2. For a comprehensive analysis of the BRCA1/2 mutation spectrum, all mutations reported in KoOC patients thus far were investigated and have been summarized in Table 3. To date, 111 mutations have been reported in KoOC patients, including 87 BRCA1 mutations (78.4%) and 24 BRCA2 mutations (21.6%) (Fig. 2A). BRCA1 mutations were more prevalent than BRCA2 mutations, with a BRCA1-to-BRCA2 prevalence ratio of 3.6:1. Of the 111 BRCA1/2 mutations, 55 distinct mutations were identified, including 39 BRCA1 and 16 BRCA2 mutations. Among these pathologic 55 mutations, 24 (43.6%) were specific to Korean women and have not been previously reported in the BIC database (Fig. 2C). Ten Korean population-specific mutations (18.2%) have not yet been reported in the KOHBRA study. Nineteen distinct mutations (19/55 distinct, 34.5%) were recurrent and were detected in at least two unrelated patients (Fig. 2D). Of these recurrent mutations, five were highly recurrent and were found more than five times in unrelated patients: c.3627insA (n=14), c.390C>A (n=8), c.922_923AGCinsT (n=5), c.5339T>C (n=5), and c.3442delG (n=5) (Table 4). These five recurrent mutations account for 33.3% (37 of 111) of the mutations detected in the Korean population. These highly recurrent mutations were considered to be most likely the founder mutations in KoOC, and all of these have been reported in the KOHBRA study (Table 4).
DiscussionIn this study, we included 279 unselected EOC patients and comprehensively evaluated BRCA1/2 mutations in the largest multicenter Korean cohort to date. We also analyzed the BRCA1/2mutation spectrum, including all mutation variants previously reported in KoOC. Across previous studies in Korea, the frequency of BRCA1/2mutations has reportedly been quite diverse, ranging between 23.8% and 31.9% [9-11] (S3 Table); this variation could have been caused by inter-study heterogeneity, including the differences in sample size, inclusion criteria, and the methods used for detecting mutation. In the present study, the overall frequency of BRCA1/2 mutations in unselected KoOC patients was 16.5% (Fig. 1A), which was comparable to the frequency of 13% to 15% in western cohorts [6,14,15]. In addition, the frequency of BRCA1/2 mutations observed in the present study was comparable to the frequencies reported in other recent Asian reports, ranging from 11% to 17% [16-18]. These data suggest that there are no marked ethnic differences in the frequency of BRCA1/2 mutations.
In terms of the age-of-onset, patients with BRCA1/2 mutation are characterized by developing EOC at a younger age, based on a report of western cohorts [6]. Unlike the data for western cohorts, in this study, the mean age of patients with BRCA1/2 mutation was not significantly lower than that of those without BRCA1/2mutation (53.7±9.6 years vs. 54.2±10.9 years, p=0.765). There were no significant differences in the proportion of patients with early age-of-onset (< 50 years) of ovarian cancer between BRCA1/2mutation carriers and non-carriers (39.1% vs. 30.5%, p=0.223) in KoOC patients. Similarly to the present study, other studies including Asian populations have also shown no significant difference in the mean age-of-onset based on the presence or absence of BRCA1/2mutation [16-18]. Difference of genetic background and non-genetic factors, such as birth cohort and reproductive and lifestyle-related factors, could have influenced different results in Asian EOC patients.
Regarding the clinicopathological findings, patients with BRCA1/2 mutations are characterized by serous histology [6,19] and advanced stage with unfavorable outcomes [6,19,20]. In the present study, 91.3% (42/46 BRCA1/2 mutation cases) had serous histology (Table 1). Only four cases (8.7%) with non-serous histology were identified, comprising three cases with clear carcinoma and one with endometrioid carcinoma. We did not find any other mucinous or mixed types (S2 Table). The fraction of serous carcinoma in KoOC patients with BRCA1/2 mutation was comparable to those previously mentioned in some western reports. Our study also found that BRCA1/2 mutations were significantly associated with an advanced FIGO stage of III/IV (p=0.007) and LN metastasis (p=0.011) (Table 1); in case of LN metastasis, it was not significant in multivariate logistic regression (Table 2).
Recently, two relatively large population-based studies including 95 and 916 women with unselected ovarian cancer were performed in Japan [17] and China [18], respectively, which are geographically close to Korea. On comparing three studies of East Asian populations, including the present Korean study, we found no marked ethnic differences in the BRCA1/2 mutation frequency among the studies (12.6% in Japan, 16.7% in China, and 16.5% in Korea) (Table 5). These East Asian studies showed no significant difference in the mean age-of-onset between BRCA1/2 mutation carriers and non-carriers (Japan, 57 years vs. 55 years; China, 53 years vs. 54 years; and Korea, 53 years vs. 54 years) (Table 5). In addition, these three studies revealed significant associations between BRCA1/2 mutation and clinicopathological characteristics, including serous histology and advanced FIGO stage (Table 5). However, compared with EOC patients with BRCA1/2 mutation in Korea and China, those in Japan showed a lower frequency of BRCA1/2 mutation and higher mean age of onset. The high frequency of BRCA2 mutations and the low proportion of FH of ovarian and/or breast cancer in Japanese EOC patients may have led to these differences in results, possibly indicating that the BRCA1/2 mutation spectrum and/or founder mutations in Japanese EOC patients markedly differ from those in the other two countries.
Although only a few single-center studies including KoOC patients have been done, a large majority of spectrum analysis of BRCA1/2mutations in Korean HBOC patients was performed by the nationwide KOHBRA study [12,13]. Among the 2,403 index patients, 378 were found to carry BRCA1/2 mutations. Overall, 153 distinct mutations were identified, including 63 in BRCA1 and 90 in BRCA2. When the BRCA1/2 mutation spectrum was compared between KoOC and KoBC patients, the BRCA1-to-BRCA2 ratio was 3.6:1.0 in KoOC and 1.0:1.4 in KoBC patients, representing that BRCA1mutations (78.4%) were predominant in KoOC (Fig. 2A), whereas BRCA2 mutations (59.2%) were predominant in KoBC (Fig. 2B). In the KOHBRA study, a replacement mutation, c.7480C>T, in the BRCA2 gene, which was identified as a Korean founder mutation through haplotype analysis by Seong et al. [21], was the most commonly detected mutation, accounting for 10.1% of all BRCA1/2 mutations (Fig. 2B). In the present study of KoOC patients, c.3627insA in the BRCA1 gene, was the most commonly detected mutation, accounting for 12.6% of all BRCA1/2 mutations (Fig. 2A), and c.7480C>T was identified in only four unrelated EOC patients, accounting for 3.6% of all BRCA1/2 mutations (Table 3). In our study, the BRCA1/2mutation spectrum varied according to the cancer type (ovarian and breast cancers) among the Korean HBOC population. The inconsistency of the BRCA1/2 mutation spectrum between ovarian cancer and breast cancer in Korean HBOC was an unexpected result. Previously, Rebbeck et al. [22] investigated hazard ratios for breast and ovarian cancer based on mutation type, function, and nucleotide position in the international sample comprised of 19,581 BRCA1/2mutation carriers from 55 centers in 33 countries on six continents. They identified three breast cancer cluster regions (BCCRs) and one ovarian cancer cluster region (OCCR) in BRCA1 and three BCCRs and three OCCRs in BRCA2, suggesting that breast and ovarian cancer risks were different by type and location of BRCA1/2 mutations. These results support possible presence of mutation sequence-associated penetrance tendency according to cancer type, breast or ovarian cancer, in hereditary KoBOC. To elucidate the likelihood of mutation sequence-related penetrance tendency leading to breast or ovarian cancer in Korean HBOC, further studies regarding pooled database and risk assessment of BRCA1/2 mutations for Korean HBOC are required.
The identification of recurrent mutations, including founder mutations, enables the development of cost-effective screening protocols and more efficient genetic counseling. In previous studies including Jewish populations [23-25], three mutations (BRCA1 c.68_69delAG, c.5266dupC, and BRCA2 c.5946delT) were identified as highly recurrent founder mutations, accounting for approximately 90% of all BRCA1/2 mutations. In fact, screening based on these three founder mutations alone is currently ongoing in clinical practice for Ashkenazi Jewish individuals. In the present study, 65.4% (36 of 55 distinct) of BRCA1/2 mutations were non-recurrent and the remaining 34.6% (19 of 55 distinct) were recurrent. Among the recurrent BRCA1/2 mutations, five highly recurrent mutations, which were the candidates for Korean founder mutations, accounted for only 33.3% (37 of 111) of mutations detected in KoOC (Table 4). Considering the diversity of mutation spectrum and the low proportion of highly recurrent mutations in KoOC patients with BRCA1/2 mutation, a screening protocol aimed at identifying highly recurrent founder mutations may not be effective. Currently, a full direct sequencing analysis for BRCA1/2 is being performed in KoOC patients for the initial screening of HBOC.
Although this study is the first multi-center study in Korea, all the participating centers are located in the city of Busan, making our study less representative of the country as a whole. In addition, the absolute number of participants carrying BRCA1/2mutations was relatively small. Nevertheless, the prevalence and clinical significance, excluding the early onset of ovarian cancer, of BRCA1/2 mutations in KoOC patients was not significantly different from the values reported in large population-based western studies. In this investigation, we found that KoOC and KoBC had different spectra of the BRCA1/2 mutations; however, indirect analyses between KoOC and KoBC patients were conducted to compare the BRCA1/2 mutation spectrum; herein, the data for KoBC patients were from the KOHBRA study. To confirm our results, further direct comparisons of BRCA1/2mutation spectra in a single cohort of Korean HBOC patients is required.
In conclusion, we demonstrated herein that KoOC patients with BRCA1/2 mutation were significantly associated with several clinicopathologic factors, such as serous histology and an advanced FIGO stage. However, KoOC patients with BRCA1/2 mutation were not characterized by an early age-of-onset (age < 50) of ovarian cancer. An analysis of the BRCA1/2 mutation spectrum in KoOC patients showed that the BRCA1-to-BRCA2 ratio and the most commonly detected mutation were different from those of KoBC patients. Owing to the different mutation spectra of KoOC and KoOC patients among Korean HBOC, there were might be presence of possible penetrance tendency leading to breast cancer or ovarian cancer by mutation sequence-specific manner. Taken together, our observations may provide an insight for better genetic counseling and clinical surveillance for the at-risk relatives of KoOC patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
AcknowledgmentsThis research was supported in part by a Biomedical Research Institute Grant (2017-01), Pusan National University Hospital and the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2016M3A9E8942067).
Fig. 1.Prevalence of BRCA1/2 mutations in unselected 279 Korean ovarian cancer patients. The overall prevalence (A) and prevalence according to family history of breast/ovarian cancer (FHBOC) (B). 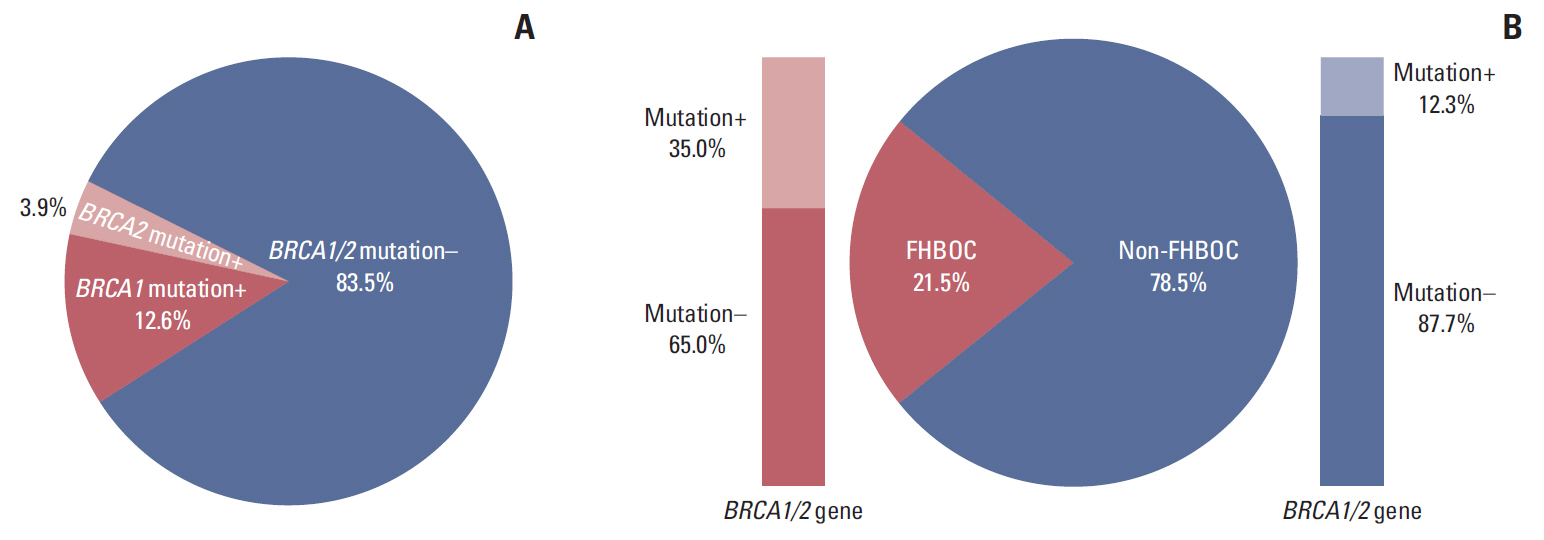
Fig. 2.Spectrum analysis of 111 (55 distinct) BRCA1/2 mutations detected in Korean ovarian cancer (KoOC) patients. The ratio of BRCA1 to BRCA2 mutations and most frequent mutation in KoOC patients (A), and the corresponding values in in Korean breast cancer patients as analyzed in the Korean Hereditary Breast Cancer (KOHBRA) study (B). The proportions of Korean population-specific mutation to non-Korean population-specific mutation (C) and recurrent mutation to non-recurrent mutation (D). 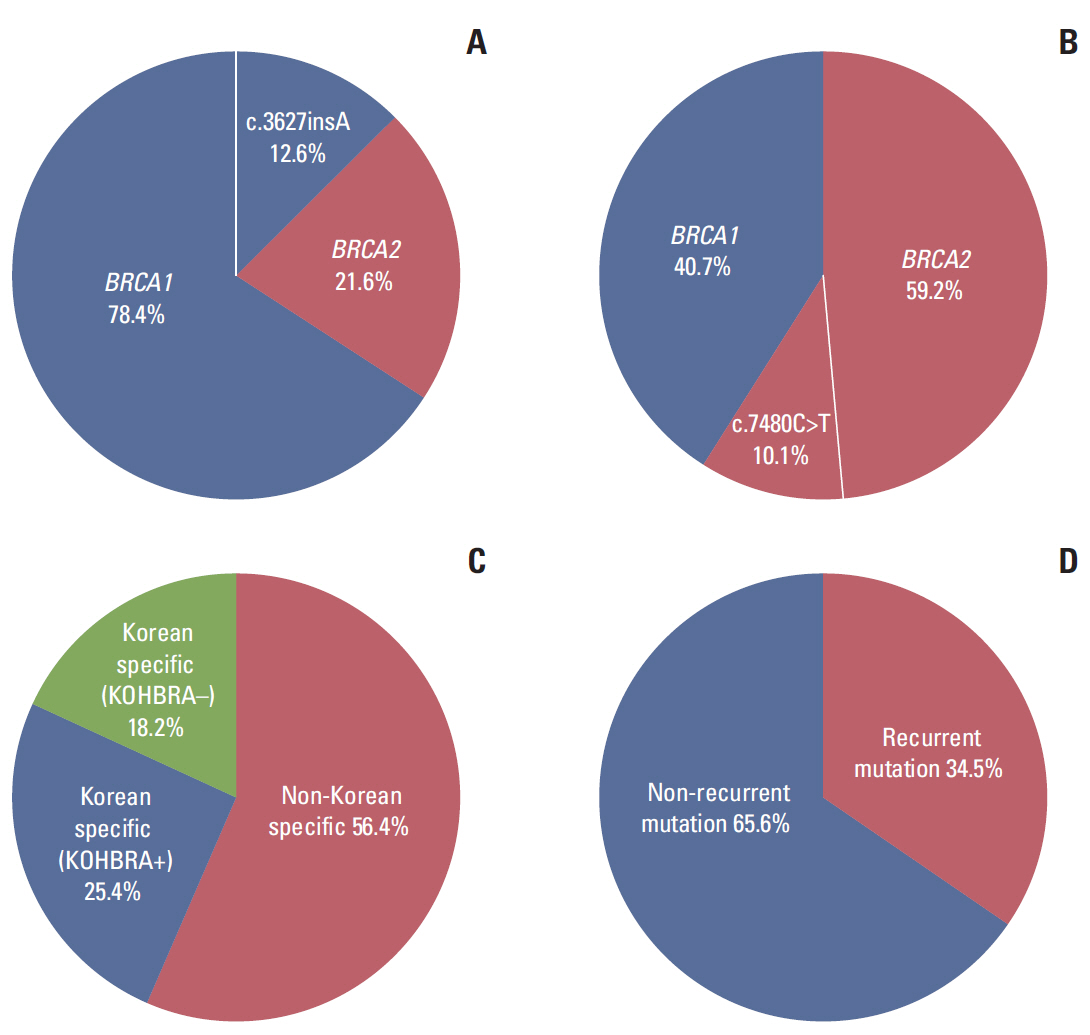
Table 1.Association of BRCA1/2 mutation status with clinicopathological characteristics in Korean ovarian cancer patients
Values are presented as number (%) or mean±standard deviation. FHBOC, family history of breast/ovarian cancer; FH, family history; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node. Table 2.Multivariate logistic regression in Korean ovarian cancer patients between BRCA1/2 mutation status and clinicopathological characteristics
Table 3.Spectrum of BRCA1/2 mutations in Korean ovarian cancer (KoOC) patients
BIC, Breast Cancer Information Core; KOHBRA, Korean Hereditary Breast Cancer; NR, not reported; R, reported; MS, missense; NS, nonsense; FS, frameshift; SP, splicing. a) Nomenclature system of the Human Genome Variation Society (http://www.hgvs.org/mutnomen), Table 4.Spectrum of highly frequent BRCA1/2 mutations in Korean hereditary breast and ovarian cancer patients
KoOC, Korean ovarian cancer; KOHBRA, Korean Hereditary Breast Cancer; FS, frameshift; NS, nonsense; MS, missense. a) Nomenclature system of the Human Genome Variation Society (http://www.hgvs.org/mutnomen), Table 5.Comparison of BRCA1/2 mutations in unselected patients with ovarian cancer from three Asian countries
References1. Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19:961–8.
2. Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016;27:e5
3. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305.
4. Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473–81.
5. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. "BRCAness" syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6.
6. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
7. Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–9.
8. Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51.
9. Lim MC, Kang S, Seo SS, Kong SY, Lee BY, Lee SK, et al. BRCA1 and BRCA2 germline mutations in Korean ovarian cancer patients. J Cancer Res Clin Oncol. 2009;135:1593–9.
10. Choi MC, Heo JH, Jang JH, Jung SG, Park H, Joo WD, et al. Germline mutations of BRCA1 and BRCA2 in Korean ovarian cancer patients: finding founder mutations. Int J Gynecol Cancer. 2015;25:1386–91.
11. Eoh KJ, Park HS, Park JS, Lee ST, Han J, Lee JY, et al. Comparison of clinical outcomes of BRCA1/2 pathologic mutation, variants of unknown significance, or wild type epithelial ovarian cancer patients. Cancer Res Treat. 2017;49:408–15.
12. Kim H, Cho DY, Choi DH, Choi SY, Shin I, Park W, et al. Characteristics and spectrum of BRCA1 and BRCA2 mutations in 3,922 Korean patients with breast and ovarian cancer. Breast Cancer Res Treat. 2012;134:1315–26.
13. Kang E, Seong MW, Park SK, Lee JW, Lee J, Kim LS, et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res Treat. 2015;151:157–68.
14. Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16.
15. Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706.
16. Hasmad HN, Lai KN, Wen WX, Park DJ, Nguyen-Dumont T, Kang PC, et al. Evaluation of germline BRCA1 and BRCA2 mutations in a multi-ethnic Asian cohort of ovarian cancer patients. Gynecol Oncol. 2016;141:318–22.
17. Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi H, Ikegami A, Teramoto K, et al. BRCA1 and BRCA2 mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer. 2016;122:84–90.
18. Shi T, Wang P, Xie C, Yin S, Shi D, Wei C, et al. BRCA1 and BRCA2 mutations in ovarian cancer patients from China: ethnic-related mutations in BRCA1 associated with an increased risk of ovarian cancer. Int J Cancer. 2017;140:2051–9.
19. Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–5.
20. Soegaard M, Kjaer SK, Cox M, Wozniak E, Hogdall E, Hogdall C, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin Cancer Res. 2008;14:3761–7.
21. Seong MW, Cho S, Noh DY, Han W, Kim SW, Park CM, et al. Comprehensive mutational analysis of BRCA1/BRCA2 for Korean breast cancer patients: evidence of a founder mutation. Clin Genet. 2009;76:152–60.
22. Rebbeck TR, Mitra N, Wan F, Sinilnikova OM, Healey S, McGuffog L, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313:1347–61.
23. Friedman LS, Szabo CI, Ostermeyer EA, Dowd P, Butler L, Park T, et al. Novel inherited mutations and variable expressivity of BRCA1 alleles, including the founder mutation 185delAG in Ashkenazi Jewish families. Am J Hum Genet. 1995;57:1284–97.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||