AbstractPurposeThis randomized phase III study was designed to compare the efficacy and safety of irinotecan plus cisplatin (IP) over etoposide plus cisplatin (EP) in Korean patients with extensive-disease small-cell lung cancer (SCLC).
Materials and MethodsPatients were randomly assigned to receive IP, composed of irinotecan 65 mg/m2 intravenously on days 1 and 8+cisplatin 70 mg/m2 intravenously on day 1 every 3 weeks, or EP, composed of etoposide 100 mg/m2 intravenously on days 1, 2, 3+cisplatin 70 mg/m2 intravenously on day 1, every 3 weeks for a maximum of six cycles, until disease progression, or until unacceptable toxicity occurred. The primary endpoint was overall survival.
ResultsA total of 362 patients were randomized to IP (n=173) and EP (n=189) arms. There were no significant differences between IP and EP arms for the median overall survival (10.9 months vs. 10.3 months, p=0.120) and the median progression-free survival (6.5 months vs. 5.8 months, p=0.115). However, there was a significant difference in response rate (62.4% vs. 48.2%, p=0.006). The pre-planned subgroup analyses showed that IP was associated with longer overall survival in male (11.3 months vs. 10.1 months, p=0.036), < 65 years old (12.7 months vs. 11.3 months, p=0.024), and Eastern Cooperative Oncology Group performance status 0/1 (12.4 months vs. 10.9 months, p=0.040) patient groups. The severity of treatment-related adverse events such as grade 3/4 anemia, nausea and diarrhea was more frequent in patients treated with IP.
IntroductionLung cancer is one of the leading causes of the cancer-related deaths [1,2]. Approximately 15% of new cases of lung cancer are diagnosed with small cell lung cancer (SCLC) [3]. Most patients with SCLC present with hematogenous metastases, and approximately two-thirds of the patients present with extensive disease. Despite the substantial initial sensitivity of SCLC to chemotherapy, a majority of patients with extensive-disease (ED) SCLC eventually die within 1 year of initial diagnosis as a result of the relapse. Thus, both new regimens and alternative doses and schedules are considered to be treatment options for patients with ED SCLC.
The platinum-based chemotherapy consisting of etoposide and cisplatin (EP) has been the standard therapy for ED-SCLC for decades [4-7]. The response rates typically achieved with EP regimen ranged from 60% to 80% with median overall survival (OS) of 8 to 10 months. Preliminary studies with irinotecan hydrochloride, a topoisomerase I inhibitor, revealed the promising outcome against SCLC and the subsequent phase II study with irinotecan and cisplatin reported a complete response rate of 29% and an overall rate of 86% in patients with ED SCLC [8,9]. Based on these initial reports, the Japanese Cooperative Oncology Group (JCOG) conducted a phase III study to compare the efficacy and toxicity of irinotecan plus cisplatin (IP) versus EP as the firstline of chemotherapy for ED-SCLC [10,11]. Median OS and 1-year survival rate were significantly higher with IP than with EP (median survival for IP vs. EP, 12.8 vs. 9.4 months, and 1-year survival rate, 58.4% vs. 37.7%). The severe toxicity in the IP arm was grade 3/4 diarrhea whereas the severe myelosuppression was observed more frequently in the EP arm.
Despite this positive role of IP for ED SCLC, two subsequent large-scale phase III trials failed to confirm the superior outcome of IP over EP in the United States, Australia, and Canada [12-14]. The cause of inconsistent result for IP regimen between Japanese and Western populations has not been elucidated yet. One possible cause is the pharmacogenetic difference of irinotecan between Asian and Western population. Based on the rationale that Korean and Japanese population might share substantial degree of pharmacogenetic profile compared to Western population, we hypothesized that the efficacy of IP in Korean patients with ED SCLC might be superior to that of EP. To test this hypothesis, we conducted the phase III trial to evaluate the efficacy and safety of IP versus EP regimen for chemotherapy naïve patients with ED SCLC in Korea.
Materials and Methods1. Study populationWe conducted a randomized, multi-center, phase III trial to compare the efficacy and safety of IP and EP for chemotherapy naïve patients with ED SCLC (ClinicalTrials.gov Identifier: NCT00-349492).
Eligible criteria were as follows: histologically or cytologically confirmed SCLC; extensive-stage disease (defined as presence of either distant metastasis, contralateral hilar lymph node involvement, or cytologically proven malignant pleural effusion); chemotherapy naïve; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; age ≥ 18 years; measurable lesions on computed tomography or magnetic resonance imaging as defined by Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.0; adequate function of bone marrow (absolute neutrophils count ≥ 1,500/mm3, white blood cell ≥ 4,000/mm3, and platelet count ≥ 100,000/mm3, hemoglobin ≥ 9.0 g/dL), liver (bilirubin level ≤ 1.5× the upper limit of normal [ULN], and aminotransferase and alanine aminotransferase ≤ 2.5×ULN or ≤ 5×ULN if liver metastases were present), and kidney (creatinine level ≤ 1.5×ULN). Patients with known brain metastases were eligible if they were asymptomatic or neurologically stable without steroids after surgery or radiotherapy.
Patients were excluded if they had another malignancy except cured basal cell carcinoma or cured uterine cervical carcinomas in situ within 5 years. The patients with the following conditions were also excluded; had a history of significant cardiovascular disease, serious lung disease, metabolic disease, and serious active infection, had impaired mental status, had enrolled in other study within 30 days, and if they were pregnant and breast-feeding.
2. Treatment and evaluationPatients were randomly assigned to receive either IP or EPand were stratified by ECOG PS (0, 1 vs. 2) and the treating institutes. The IP regimen consisted of irinotecan 65 mg/m2 intravenously on days 1 and 8 and cisplatin 70 mg/m2 intravenously on day 1 every 3 weeks. The EP regimen consisted of etoposide 100 mg/m2 intravenously on days 1, 2, 3 and cisplatin 70 mg/m2 intravenously on day 1 every 3 weeks. Treatment in each arm was repeated for a maximum of six cycles. Primary prophylactic use of granulocyte-colony stimulating factor (G-CSF) was not allowed before the first cycle of treatment, but secondary prophylactic use of G-CSF after neutropenia was allowed. Prophylactic cranial irradiation after completion of study treatment and crossover treatment after disease progression were allowed based upon investigator’s discretion.
3. Statistical methodsThe primary objective of this study was to compare the OS in patients with extensive stage small-cell lung cancer treated with EP (standard arm) with that in comparable patients treated with the IP (experimental). IP would be judged superior to the standard if the true increase in median OS was 3.0 months. We used Freedman’s sample size formula for log-rank statistic assuming that the hazard ratio (HR) is constant throughout the trial [17]. A total of 333 events were required to demonstrate a significant superiority of OS with an α of 5% and a power of 80% at final analysis, using a one-sided stratified log-rank test. Assuming that the expected survival rate will be 5.2% at 18 months in the standard arm, a total of 362 patients were calculated. Planned interim analysis was conducted at the time of 166 events (50% of target events) occurred. α1=0.0055 and α2=0.0482 were used by O’Brien Flemming method. OS and progression-free survival (PFS) were calculated using the Kaplan-Meier method and log-rank test was employed to compare survival rates. HR was presented together with the 90% two-sided confidence interval. OS was calculated from the day of start of treatment until death by any cause; surviving patients were censored at the last date of follow-up. PFS was calculated from the day of treatment until disease progression or death from any cause. Efficacy was analyzed on intention-to-treat population. Exploratory subgroup analysis was planned to be conducted by considering factors such as sex, age, and ECOG status.
4. Ethical statementThe study was approved by the Institutional Review Boards of participating institutions (Seoul National University Hospital, Seoul; Gyeongsang National University Hospital, Jinju; Yonsei Cancer Center, Yonsei University College of Medicine, Seoul; Samsung Medical Center, Seoul; St. Vincent’s Hospital, The Catholic University of Korea, Suwon; Chung-Ang University College of Medicine, Seoul; Veterans Health Service Medical Center, Seoul; Seoul St. Mary’s Hospital, Seoul; Yeungnam University Medical Center, Daegu; Asan Medical Center, Seoul; Daegu Catholic University Hospital, Daegu; SMG-SNU Boramae Medical Center, Seoul; Chungbuk National University Hospital, Chungju; Hallym University College of Medicine Kangdong Sacred Heart Hospital, Seoul; Ajou University Hospital, Suwon; Korea University Anam Hospital, Seoul; Soon Chun Hyang University Bucheon Hospital, Bucheon) and was conducted in compliance with Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Results1. Patients and treatmentThe characteristics of the patients are listed in Table 1. Most patients (> 85%) were male. The median age for IP and EP arm was 66 years (range, 47 to 80 years) and 65 years (range, 36 to 81 years), respectively. More than 80% of patients had an ECOG PS of 0 or 1. More than 60% of patients had metastases to the bone and brain metastases were detected in about 25% of patients. Baseline characteristics were well balanced between arms.
From June 2006 to November 2011, 362 patients were enrolled in this study at 19 sites with 173 patients randomly assigned to receive IP and 189 patients randomly assigned to receive EP (S1 Fig.). All patients who received at least one cycle of study treatment were assessable for the efficacy and toxicity analyses. Six and two patients in IP and EP arm were given no chemotherapy because they didn’t meet the enrollment criteria. These patients were excluded from the toxicity analysis. For the remaining 167 patients in the IP arm, the mean dose intensities of irinotecan and cisplatin were 92.6% and 95.4% (irinotecan, 120.4 mg/m2/wk of 130 mg/m2/wk and cisplatin, 66.8 mg/m2/wk of 70 mg/m2/wk) of the planned doses, respectively. For the remaining 187 patients in the EP arm, the mean dose intensities of EP were 96.1% and 97% (etoposide, 288.5 mg/m2/wk of 300 mg/m2/wk and cisplatin, 67.9 mg/m2/wk of 70 mg/m2/wk) of the planned doses, respectively. There were no significant differences between two arms in treatment delivery.
The number of the patients who completed the study was 97 (56%) and 98 (51%) in the IP and EP arm, respectively. The most common reason for not completing study was due to progressive disease, treatment-related complications, unacceptable toxicity, and deaths. The median number of cycles of chemotherapy administered was 6 in each arm; more than 96 % of patients on the IP and EP arm received at least one study treatment. The duration of follow-up time was 12.5 months in the IP arm and 11.4 months in the EP arm.
2. Survival and tumor responseThere was no significant difference between two arms in OS (median OS, 10.9 vs. 10.3 months; p=0.120) and PFS (median PFS, 6.5 vs. 5.8 months; p=0.115), The Kaplan-Meier curves of OS and PFS of the assessable patients are shown in Figs. 1 and 2. The 1-year and 2-year OS rates between two arms were also similar. This result indicates that there is no significant difference in survival rate between two arms. In the pre-defined exploratory subgroup analysis (Fig. 3), however, HRs for OS seemed to favor the IP treatment arm in male (11.3 months vs. 10.1 months, p=0.036), < 65 years old (12.7 months vs. 11.3 months, p=0.024), and ECOG performance status 0/1 (12.4 months vs. 10.9 months, p=0.040) patient groups. There was a significant prolongation of survival in the IP over EP regimen within these patient groups.
Tumor response data are shown in Table 2. In the IP and EP arms, respectively, 24 (13.9%) and 51 (30%) experienced stable disease, and 2.3% and 9.5% experienced progressive disease. There were two complete responses (1.1%) and 106 partial responses (61.3%) in the IP arm while there were three complete responses (1.6%) and 88 partial responses (46.6%) in the EP arm. There was a significant difference in objective response rate between IP and EP arms (p=0.006); Objective response rate in the IP arm was 62.4% and 48.4% in the EP arm.
3. ToxicityGrade ≥ 3 adverse events which occurred in more than 2% of subjects are summarized in Table 3. The most common toxicity with grade ≥ 3 or more was neutropenia; 104 patients (62.3%) and 134 patients (71.7%) experienced grade ≥ 3 neutropenia in the IP and EP arm, respectively. Significantly higher rates of grade ≥ 3 anemia, nausea, and diarrhea occurred in the IP arm compared with the EP arm. There were nine and 10 treatment-related deaths in the IP and EP arm, respectively. Toxicities resulting in discontinuation of study treatment occurred in 17 subjects in the IP arm and 22 in the EP arm.
4. Systemic treatments after progressionSystemic treatments after progression were summarized in Table 4. The proportion of patients who received additional treatment was similar between two arms. Among the patients who received the post-progression systemic treatment, 48.6% of patients in the IP arm received subsequent treatment containing etoposide and 34.4% of those in the EP arm received subsequent treatment containing irinotecan.
DiscussionResults from our study showed no significant difference in the OS in the IP arm, as shown by the median survival time of 10.9 and 10.3 months in the IP and EP arm, respectively. Our results are summarized in Table 5 together with those from other similar studies in the literature. However, the pre-defined exploratory subgroup analysis demonstrated significant benefit of IP over EP in male, < 65 years old, and ECOG PS 0/1 patient groups, indicating that IP regimen might be favorable for these particular groups. There was some imbalance noted in the gender distribution between two treatment groups, with a greater proportion of male in the EP arm by 7% (p=0.038). However, such imbalance dose not influence the overall non-significant treatment difference after adjustment (p=0.121). The objective response rate from our study was also significantly higher in IP arm than EP arm. As in agreement with previous studies, grade 3/4 neutropenia was most frequent in both arms but not significantly different between two arms. However, severe toxicities such as grade 3/4 anemia, nausea, and diarrhea were significantly higher in the IP arm than in the EP arm.
Although there has been the controversy regarding the superiority of IP regimen for ED SCLC, our study suggests that IP regimen might not be superior to EP regimen, the current standard chemotherapy for ED SCLC patients. Several factors such as treated doses and schedules of regimens, patient characteristics, post-study treatment might be responsible for the divergent result between trials. Much attention, however, has been paid to the distinct pharmacogenetics existing between different ethnic populations [18-20]. It is well established that the single-nucleotide polymorphisms (SNPs) of genes involved in the drug disposition vary among ethnic populations. Lara et al. [12] reported that certain SNPs such as ABCB1 (C3435T)T/T and UGT1A1 (G-3156A)A/A seemed to be associated with specific toxicities including diarrhea and neutropenia, but they failed to detect any other genotypes correlated with the treatment efficacy. Intriguingly, however, Han et al. [21] found that ABCC224TT and 3972TT genotypes were associated with higher response rates and longer PFS in Korean patients with advanced non-SCLC. Thus, further ongoing research on the pharmacogenetics is eagerly awaited to further explain the inter-individual and inter-ethnic variability in efficacy and toxicity of chemotherapy. In addition to the role of pharmacogenetics, other genetic alterations might serve as important predictors for response and toxicity to irinotecan. For example, the expression profiling analysis using irinotecan-sensitive and resistant SCLC cell lines might facilitate the identification of novel genes involved in various responses to chemotherapy regimen.
It was of note that, according to our subgroup analysis, IP might be a beneficial treatment option for patient groups with a good performance status. This implies that irinotecan/cisplatin combination might be useful for treating patients with limited-disease (LD) SCLC. Indeed, IP has been tested in randomized phase II trials in patients with LD SCLC. Sohn et al. [22] reported that IP with early concurrent radiotherapy was effective and tolerable in untreated LD-SCLC. Another phase II study of IP induction followed by concurrent twice-daily thoracic radiotherapy with EP chemotherapy for LD SCLC also showed the encouraging results [23]. However, the subsequent phase III trial comparing EP versus IP in patients with LD SCLC treated with EP plus concurrent accelerated hyperfractionated thoracic radiotherapy did not show the improved outcome from IP compared with EP regimen in LD SCLC [24]. Thus, further study is needed to validate IP regimen before recommended as the standard chemotherapy for LD SCLC.
Although the confounding effects of crossover treatment could be a limiting factor to be considered in our study, this study shows that the irinotecan/cisplatin combination as the first-line chemotherapy is not superior to etoposide/cisplatin combination. Furthermore, the toxicity profile of IP was rather unfavorable to EP overall. Therefore, IP might be an alternative treatment option for patients who experience the recurrence after receiving the EP-based first line of chemotherapy.
In conclusion, the IP arm did not show the superior efficacy compared with the EP arm in patients with ED SCLC in Korean patients. Grade 3/4 toxicity was more common in IP arm than in EP arm. However, IP chemotherapy might be beneficial comparing with EP chemotherapy in male, < 65 years old, and ECOG PS 0/1 patient subgroups, indicating that IP regimen might be favorable for these particular groups.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
AcknowledgmentsWe thank the participating patients and their families, all study co-investigators, and research coordinators. We also thank Ms. Soohee Kang (Medical Research Collaboration Center, Seoul National University Hospital, Seoul, Republic of Korea) for the support in statistical analyses. Medical writing assistance was provided by Seonah Ha Ph.D. This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A040151).
Fig. 1.Kaplan-Meier curve of overall survival. IP, irinotecan/cisplatin; EP, etoposide/cisplatin; HR, hazard ratio; CI, confidence interval. 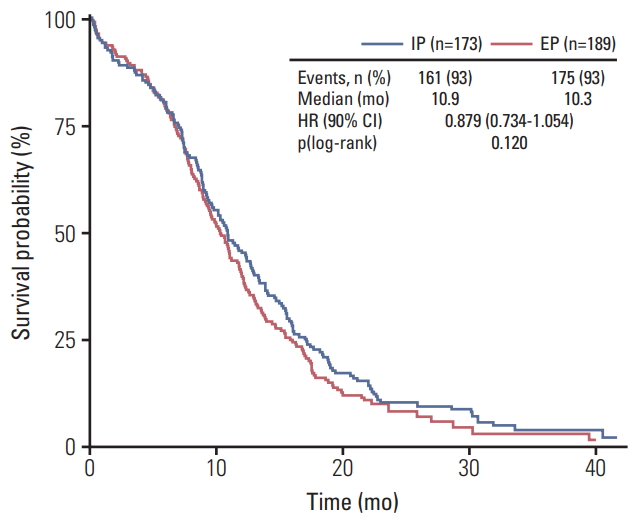
Fig. 2.Kaplan-Meier curve of progression-free survival. IP, irinotecan/cisplatin; EP, etoposide/cisplatin; HR, hazard ratio; CI, confidence interval. 
Fig. 3.Standard forest plot of the hazard ratio for overall survival according to the pre-defined subgroups. HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IP, irinotecan/cisplatin; EP, etoposide/cisplatin. 
Table 1.Baseline characteristics
Table 2.Best overall response
Table 3.Grade ≥ 3 adverse events in more than 2% of subjects
Table 4.Systemic treatments after progression Table 5.Comparison of relevant studies of irinotecan-based chemotherapy in patients with ED SCLC
References1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305.
4. Roth BJ, Johnson DH, Einhorn LH, Schacter LP, Cherng NC, Cohen HJ, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282–91.
5. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: small cell lung cancer (version 1) [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; 2015. [cited 2018 Mar 8]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
6. Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi99–105.
7. Lebeau B, Chastang C, Allard P, Migueres J, Boita F, Fichet D. Six vs twelve cycles for complete responders to chemotherapy in small cell lung cancer: definitive results of a randomized clinical trial. The "Petites Cellules" Group. Eur Respir J. 1992;5:286–90.
8. Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992;10:1225–9.
9. Kudoh S, Fujiwara Y, Takada Y, Yamamoto H, Kinoshita A, Ariyoshi Y, et al. Phase II study of irinotecan combined with cisplatin in patients with previously untreated small-cell lung cancer. West Japan Lung Cancer Group. J Clin Oncol. 1998;16:1068–74.
10. Negoro S, Noda K, Nishiwaki Y, Kawahara M, Tamura T, Sugiura T, et al. A randomized phase III study of irinotecan and cisplatin (CP) versus etoposide and cisplatin (EP) in extensive-disease small-cell lung cancer (ED-SCLC): Japan Clinical Oncology Group Study (JCOG 9511). Lung Cancer. 2000;29(Suppl 1):S30.
11. Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91.
12. Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–5.
13. Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43.
14. Lara PN Jr, Chansky K, Shibata T, Fukuda H, Tamura T, Crowley J, et al. Common arm comparative outcomes analysis of phase 3 trials of cisplatin+irinotecan versus cisplatin+etoposide in extensive stage small cell lung cancer: final patientlevel results from Japan Clinical Oncology Group 9511 and Southwest Oncology Group 0124. Cancer. 2010;116:5710–5.
15. Common Toxicity Criteria Manual, Common Toxicity Criteria, version 3 [Internet]. Rockville, MD: National Cancer Institute; 2006. [cited 2018 Mar 8]. Available from: http://ctep.cancer.gov/reporting/ctc.html
16. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
17. Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–9.
18. Shimoyama S. Pharmacogenetics of irinotecan: An ethnicity-based prediction of irinotecan adverse events. World J Gastrointest Surg. 2010;2:14–21.
19. de Jong FA, Marsh S, Mathijssen RH, King C, Verweij J, Sparreboom A, et al. ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10:5889–94.
20. Hoskins JM, Marcuello E, Altes A, Marsh S, Maxwell T, Van Booven DJ, et al. Irinotecan pharmacogenetics: influence of pharmacodynamic genes. Clin Cancer Res. 2008;14:1788–96.
21. Han JY, Lim HS, Yoo YK, Shin ES, Park YH, Lee SY, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110:138–47.
22. Sohn JH, Moon YW, Lee CG, Kim GE, Chung KY, Chang J, et al. Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer. 2007;109:1845–950.
23. Han JY, Cho KH, Lee DH, Kim HY, Kim EA, Lee SY, et al. Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol. 2005;23:3488–94.
24. Kubota K, Hida T, Ishikura S, Mizusawa J, Nishio M, Kawahara M, et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol. 2014;15:106–13.
|
|